Tailoring the product selectivity of electrochemical CO2 reduction at copper-tin composite oxide nanofibers
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
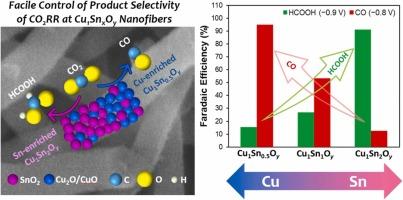
Electrochemical reduction of carbon dioxide (CO2RR) has been receiving attention as an attractive technique to convert CO2 into various useful resources. Because CO2RR generally produces diverse products and competes with hydrogen reduction reaction (HER), the development of efficient electrocatalysts exhibiting high product selectivity is required. This paper demonstrates a simple approach to synthesize Cu-Sn bimetallic oxide nanofibers with various Cu/Sn composition ratios using electrospinning and post-calcination. The prepared nanofibers (denoted as Cu1SnxOy, x = 0.5, 1, 2) showed excellent electrocatalytic activity and product selectivity for CO2RR, which were drastically improved from those of single metal oxides (i.e, CuO and SnO2). Of great importance, the product selectivity could be finely controlled by changing the Cu/Sn content ratio in Cu1SnxOy nanofibers. In fact, Cu-enriched Cu1Sn0.5Oy showed nearly exclusive faradaic efficiency (FE) for carbon monoxide (CO) at −0.8 VRHE (∼95 %); and Sn-enriched Cu1Sn2Oy exhibited high FE for formic acid at −0.9 VRHE (∼91 %), supporting the exceptionally high selectivity to CO and formic acid, respectively. In addition, both Cu1Sn0.5Oy and Cu1Sn2Oy nanofibers barely generated hydrogen during CO2RR, suppressing HER successfully.
铜锡复合氧化物纳米纤维电化学CO2还原产物选择性的研究
电化学还原二氧化碳(CO2RR)作为一种将二氧化碳转化为各种有用资源的有吸引力的技术受到了广泛的关注。由于CO2RR通常会产生多种产物,并与氢还原反应(HER)竞争,因此需要开发具有高产物选择性的高效电催化剂。本文介绍了采用静电纺丝和煅烧两种方法合成不同铜锡比的Cu-Sn双金属氧化物纳米纤维的方法。所制备的纳米纤维(记为Cu1SnxOy, x = 0.5, 1,2)对CO2RR表现出优异的电催化活性和产物选择性,较单一金属氧化物(即CuO和SnO2)有显著提高。重要的是,通过改变Cu1SnxOy纳米纤维中的Cu/Sn含量比,可以很好地控制产物的选择性。事实上,富集cu的Cu1Sn0.5Oy在−0.8 VRHE下对一氧化碳(CO)表现出几乎完全的法拉第效率(FE) (~95%);富锡Cu1Sn2Oy在−0.9 VRHE(~91%)下对甲酸表现出较高的FE,分别支持对CO和甲酸的高选择性。此外,Cu1Sn0.5Oy和Cu1Sn2Oy纳米纤维在CO2RR过程中几乎没有产生氢,成功地抑制了HER。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


