Ru3+-doped lead-free perovskite Cs3Bi2Br9 for photocatalytic selective oxidation of sulfides to sulfoxides using trace water as an oxygen source
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
It remains great challenges to keep long-term photocatalytic performance of metal halide perovskites (MHPs) under oxygen-rich and moisture environments owing to their intrinsic lability to these conditions. Herein, we employed high oxyphilic trivalent ruthenium ions (Ru3+) as oxygen atom anchors to dope into the lattice of lead-free perovskite Cs3Bi2Br9 (CBB), aiming to enhance the structural stability by inhibiting the penetration of reactive oxygen species (ROS) and water (H2O) into CBB. Besides, the photocatalytic performance of Ru3+-doped CBB (Ru-CBB) for the selective oxidation of sulfides was evaluated in the presence of O2 and H2O. Series of sulfides (38 examples) were converted into corresponding sulfoxides with excellent yields and the Ru-CBB were recycled at least 5 times without the change of crystalline structure. The enhanced photocatalytic activity of Ru-CBB, compared to the pristine CBB, can be attributed to its more positive valence band position and more efficient formation of ROS. Mechanism studies revealed that two reaction pathways, predominated by H2O and ROS respectively, were involved in the titled transformation. In the former, H2O served as an essential oxygen source for the formation of sulfoxides as established by isotope labelling experiment.

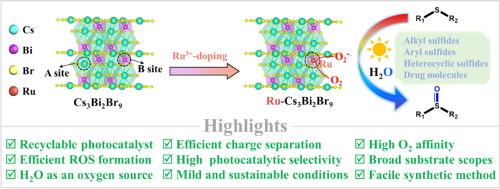
Ru3+掺杂无铅钙钛矿Cs3Bi2Br9以微量水为氧源光催化选择性氧化硫化物生成亚砜
由于金属卤化物钙钛矿(MHPs)对这些条件的固有不稳定性,在富氧和潮湿环境下保持长期的光催化性能仍然是一个巨大的挑战。本文采用高亲氧性三价钌离子(Ru3+)作为氧原子锚点,将其掺杂到无铅钙钛矿Cs3Bi2Br9 (CBB)的晶格中,旨在通过抑制活性氧(ROS)和水(H2O)对CBB的渗透来增强结构稳定性。此外,在O2和H2O的存在下,评价了Ru3+掺杂的CBB (Ru-CBB)对硫化物选择性氧化的光催化性能。一系列的硫化物(38个例子)以优异的产率转化为相应的亚砜,Ru-CBB至少可回收5次而不改变晶体结构。与原始CBB相比,Ru-CBB的光催化活性增强可归因于其更正价带位置和更有效的ROS形成。机理研究表明,这一转化过程涉及两条反应途径,分别以H2O和ROS为主。前者通过同位素标记实验证实H2O是亚砜形成的必需氧源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


