Hydrogen-based direct reduction of vanadium-titanium magnetite raw ore: Process optimization and mechanism insights
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
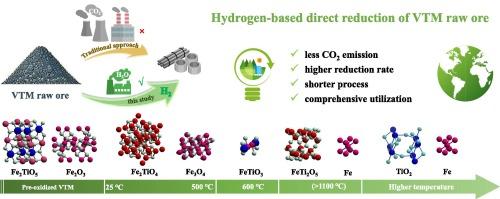
The conventional processing of vanadium-titanium magnetite (VTM) has been associated with substantial CO2 emissions and inefficient resource utilization, raising significant sustainability concerns. This study introduced a hydrogen-based direct reduction method for processing VTM raw ore without the need for a beneficiation process. Through thermodynamic analysis and experimental validation, we demonstrated the feasibility of this novel approach, achieving a remarkable metallization degree of iron up to 92.6 % at 1120 °C. Additionally, this method effectively enriched iron and titanium, yielding recovery efficiencies of 91.0 % for iron and 58.4 % for titanium via wet grinding and magnetic separation. The reduction process of VTM followed a stepwise reaction mechanism, characterized by distinct pathways for titanium and iron. The conversion sequences were as follows: Fe2TiO5 → Fe2TiO4 → FeTiO3 → FeTi2O5 → TiO2 → TixOy for titanium and Fe2O3 → Fe3O4 → FeTiO3 → Fe + FeTi2O5 → Fe for iron. Notably, the conversion of FeTiO3 to Fe emerged as the rate-limiting step, with an apparent activation energy of 75.56 kJ·mol−1. These findings underscore the potential of hydrogen-based reduction as a sustainable and resource-efficient pathway for VTM processing, significantly contributing to reducing the environmental impact of metallurgical practices.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


