Real-world effectiveness and safety of bulevirtide monotherapy for up to 96 weeks in patients with HDV-related cirrhosis
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background and aims
Bulevirtide (BLV) 2 mg/day is EMA approved for treatment of compensated chronic hepatitis due to Delta virus (HDV) infection, however real-life data in large cohorts of patients with cirrhosis are lacking.Methods
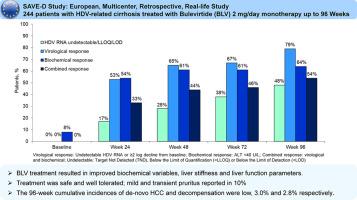
Consecutive HDV-infected patients with cirrhosis starting BLV 2 mg/day since September 2019 were included in a European retrospective multicenter real-life study (SAVE-D). Patient characteristics before and during BLV treatment were collected. Virological, biochemical, combined responses, adverse events and liver-related events (HCC, decompensation, liver transplant) were assessed.Results
244 patients with HDV-related cirrhosis receiving BLV monotherapy for a median of 92 (IQR 71-96) weeks were included: at BLV start, median (IQR) age was 49 (40-58) years, 61% men, ALT 80 (55-130) U/L, liver stiffness measurement (LSM) 18.3 (13.0-26.3) kPa, platelets 94 (67-145) x 103/mm3, 54% with esophageal varices, 95% Child Pugh score A, 10% HIV-coinfected, 92% on NUC, median HDV RNA 5.4 (4.1-6.5) Log10 IU/mL, HBsAg 3.8 (3.4-4.1) Log10 IU/mL. At weeks (W)48 and 96, virological, biochemical and combined responses were observed in 65% and 79%, 61% and 64%, 44% and 54% of patients, respectively. AST, GGT, albumin, IgG and LSM values significantly improved throughout treatment. Serum bile acid levels increased in most patients, only 10% patients reported mild and transient pruritus, independently of bile acid levels. The W96 cumulative risk of de-novo HCC and decompensation was 3.0% (95% CI 2-6%) and 2.8% (95% CI 1-5%), respectively. Thirteen (5%) patients underwent liver transplantation (n=11 for HCC, n=2 for decompensation).Conclusion
BLV 2 mg/day monotherapy up to 96 weeks was safe and effective in patients with HDV-related cirrhosis. Virological and clinical responses increased over time. Liver-related complications were few.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


