The gut–brain axis underlying hepatic encephalopathy in liver cirrhosis
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Up to 50–70% of patients with liver cirrhosis develop hepatic encephalopathy (HE), which is closely related to gut microbiota dysbiosis, with an unclear mechanism. Here, by constructing gut–brain modules to assess bacterial neurotoxins from metagenomic datasets, we found that phenylalanine decarboxylase (PDC) genes, mainly from Ruminococcus gnavus, increased approximately tenfold in patients with cirrhosis and higher in patients with HE. Cirrhotic, not healthy, mice colonized with R. gnavus showed brain phenylethylamine (PEA) accumulation, along with memory impairment, symmetrical tremors and cortex-specific neuron loss, typically found in patients with HE. This accumulation of PEA was primarily driven by decreased monoamine oxidase-B activity in both the liver and serum due to cirrhosis. Targeting PDC or PEA reversed the neurological symptoms induced by R. gnavus. Furthermore, fecal microbiota transplantation from patients with HE to germ-free cirrhotic mice replicated these symptoms and further corroborated the efficacy of targeting PDC or PEA. Clinically, high baseline PEA levels were linked to a sevenfold increased risk of HE after intrahepatic portosystemic shunt procedures. Our findings expand the understanding of the gut–liver–brain axis and identify a promising therapeutic and predictive target for HE. The gut microbiota bacterium Ruminococcus gnavus is implicated in the development of cirrhotic liver encephalopathy through the production of neurotoxins.

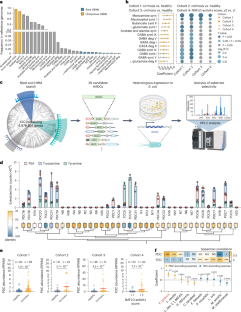
肝硬化肝性脑病的肠脑轴
高达50-70%的肝硬化患者发生肝性脑病(HE),其与肠道菌群失调密切相关,机制尚不清楚。在这里,通过构建肠-脑模块来评估来自宏基因组数据集的细菌神经毒素,我们发现苯丙氨酸脱羧酶(PDC)基因在肝硬化患者中增加了大约10倍,在HE患者中增加更多。在患有肝硬化、不健康的鼠身上,植有格纳氏鼠的小鼠表现出脑苯乙胺(PEA)积聚,同时伴有记忆障碍、对称震颤和皮层特异性神经元丢失,这些都是HE患者的典型症状。PEA的积累主要是由于肝硬化导致肝脏和血清中单胺氧化酶- b活性降低所致。以PDC或PEA为靶点可逆转地鼠引起的神经系统症状。此外,HE患者的粪便微生物群移植到无菌肝硬化小鼠身上复制了这些症状,并进一步证实了靶向PDC或PEA的有效性。临床上,高基线PEA水平与肝内门静脉分流术后HE风险增加7倍有关。我们的发现扩大了对肠-肝-脑轴的理解,并确定了一个有希望的HE治疗和预测靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
文献相关原料
公司名称
产品信息
阿拉丁
2-PhenylethylaMine
阿拉丁
metronidazole
阿拉丁
neomycin
阿拉丁
ampicillin
阿拉丁
vancomycin
阿拉丁
tyrosine
阿拉丁
phenylalanine
阿拉丁
tryptophan
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


