A multi-kinase inhibitor screen identifies inhibitors preserving stem-cell-like chimeric antigen receptor T cells
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
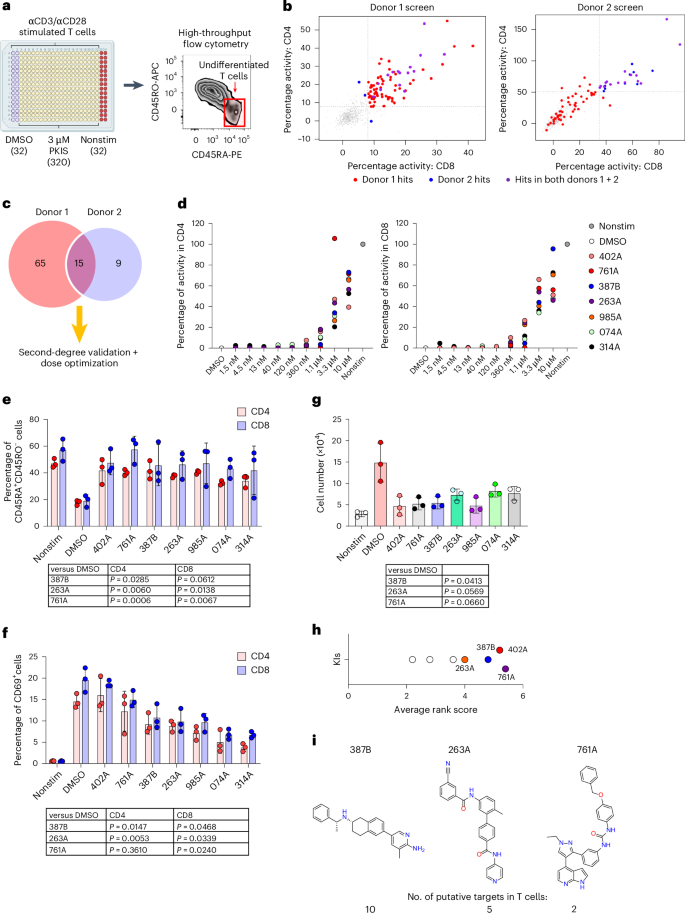
Chimeric antigen receptor T cells (CAR T cells) with T stem (TSCM) cell-like phenotypic characteristics promote sustained antitumor effects. We performed an unbiased and automated high-throughput screen of a kinase-focused compound set to identify kinase inhibitors (KIs) that preserve human TSCM cell-like CAR T cells. We identified three KIs, UNC10225387B, UNC10225263A and UNC10112761A, that combined in vitro increased the frequency of CD45RA+CCR7+TCF1hi TSCM cell-like CAR T cells from both healthy donors and patients with cancer. KI-treated CAR T cells showed enhanced antitumor effects both in vitro and in vivo in mouse tumor models. The KI cocktail maintains TSCM cell-like phenotype preferentially in CAR T cells originating from naive T cells and causes transcriptomic changes without arresting T cell activation or modulating the chromatin organization. Specific kinases, ITK, ADCK3, MAP3K4 and CDK13, targeted by the KI cocktail in a dose-dependent manner are directly associated with the preservation of TSCM cell-like CAR T cells. Knockdown of these kinases individually or in combination enriches for TSCM cell-like CAR T cells, but only CAR T cells generated in the presence of the KI cocktail show robust expansion and differentiation on stimulation with tumor cells. Overall, transient pharmacological inhibition of strategically targeted kinases maintains stem-like features in CAR T cells and improves their antitumor activity. Dotti and colleagues performed an unbiased, high-throughput screen of kinase inhibitors to identify a three-kinase inhibitor cocktail capable of preserving a stem-cell-like subset in chimeric antigen receptor T cells.

多激酶抑制剂筛选确定保留干细胞样嵌合抗原受体T细胞的抑制剂
嵌合抗原受体T细胞(CAR - T细胞)具有T干细胞(TSCM)细胞样表型特征,促进持续的抗肿瘤作用。我们对一组激酶聚焦化合物进行了无偏和自动化的高通量筛选,以鉴定保存人类TSCM细胞样CAR - T细胞的激酶抑制剂(KIs)。我们鉴定了三个KIs, UNC10225387B, UNC10225263A和UNC10112761A,它们在体外联合增加了来自健康供体和癌症患者的CD45RA+CCR7+TCF1hi TSCM细胞样CAR - T细胞的频率。在小鼠肿瘤模型中,ki处理的CAR - T细胞在体外和体内均显示出增强的抗肿瘤作用。KI鸡尾酒在源自初始T细胞的CAR - T细胞中优先维持TSCM细胞样表型,并在不阻止T细胞激活或调节染色质组织的情况下引起转录组变化。KI鸡尾酒以剂量依赖的方式靶向的特异性激酶ITK、ADCK3、MAP3K4和CDK13与TSCM细胞样CAR - T细胞的保存直接相关。这些激酶的单独或联合敲低可丰富TSCM细胞样CAR - T细胞,但只有在KI鸡尾酒存在下产生的CAR - T细胞在肿瘤细胞刺激下表现出强劲的扩增和分化。总的来说,战略性靶向激酶的短暂药物抑制维持了CAR - T细胞的干细胞样特征,并提高了它们的抗肿瘤活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


