β-Glucan reprograms neutrophils to promote disease tolerance against influenza A virus
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
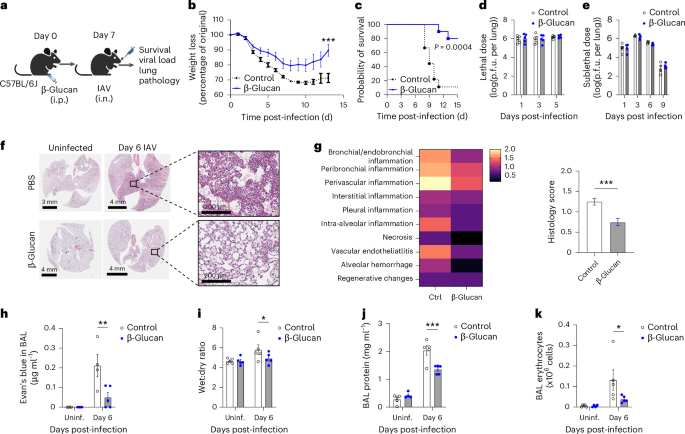
Disease tolerance is an evolutionarily conserved host defense strategy that preserves tissue integrity and physiology without affecting pathogen load. Unlike host resistance, the mechanisms underlying disease tolerance remain poorly understood. In the present study, we investigated whether an adjuvant (β-glucan) can reprogram innate immunity to provide protection against influenza A virus (IAV) infection. β-Glucan treatment reduces the morbidity and mortality against IAV infection, independent of host resistance. The enhanced survival is the result of increased recruitment of neutrophils via RoRγt+ T cells in the lung tissue. β-Glucan treatment promotes granulopoiesis in a type 1 interferon-dependent manner that leads to the generation of a unique subset of immature neutrophils utilizing a mitochondrial oxidative metabolism and producing interleukin-10. Collectively, our data indicate that β-glucan reprograms hematopoietic stem cells to generate neutrophils with a new ‘regulatory’ function, which is required for promoting disease tolerance and maintaining lung tissue integrity against viral infection. Divangahi and colleagues report that β-glucan pretreatment before influenza A virus challenge can increase survival by inducing long-term reprogramming of neutrophils, promoting disease tolerance irrespective of viral load.

β-葡聚糖重编程中性粒细胞以促进对甲型流感病毒的疾病耐受性
疾病耐受性是一种进化上保守的宿主防御策略,它在不影响病原体负荷的情况下保持组织完整性和生理机能。与宿主抗性不同,疾病耐受性的机制仍然知之甚少。在本研究中,我们研究了一种佐剂(β-葡聚糖)是否可以重新编程先天免疫,以提供对甲型流感病毒(IAV)感染的保护。β-葡聚糖治疗可降低IAV感染的发病率和死亡率,不受宿主耐药性的影响。生存率的提高是通过肺组织中的r γ T + T细胞募集中性粒细胞增加的结果。β-葡聚糖处理以1型干扰素依赖的方式促进颗粒生成,导致利用线粒体氧化代谢产生一种独特的未成熟中性粒细胞亚群并产生白细胞介素-10。总的来说,我们的数据表明β-葡聚糖重编程造血干细胞以产生具有新的“调节”功能的中性粒细胞,这是促进疾病耐受性和维持肺组织完整性免受病毒感染所必需的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


