Clec12a controls colitis by tempering inflammation and restricting expansion of specific commensals
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Microbiota composition regulates colitis severity, yet the innate immune mechanisms that control commensal communities and prevent disease remain unclear. We show that the innate immune receptor, Clec12a, impacts colitis severity by regulating microbiota composition. Transplantation of microbiota from a Clec12a−/− animal is sufficient to worsen colitis in wild-type mice. Clec12a−/− mice have expanded Faecalibaculum rodentium, and treatment with F. rodentium similarly exacerbates disease. However, Clec12a−/− animals are resistant to colitis development when rederived into an 11-member community, underscoring the role of specific species. Colitis in Clec12a−/− mice is dependent on monocytes, and cytokine and sequencing analysis in Clec12a−/− macrophages and serum shows enhanced inflammation with a reduction in phagocytic genes. F. rodentium specifically binds to Clec12a, and Clec12a−/−-deficient macrophages are impaired in their ability to phagocytose F. rodentium. Thus, Clec12a contributes to an innate-immune-surveillance mechanism that controls the expansion of potentially harmful commensals while limiting inflammation.
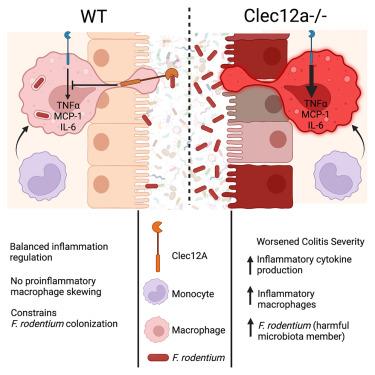
Clec12a通过缓和炎症和限制特定共生体的扩张来控制结肠炎
微生物群组成调节结肠炎的严重程度,但控制共生群落和预防疾病的先天免疫机制仍不清楚。我们发现先天免疫受体Clec12a通过调节微生物群组成影响结肠炎的严重程度。移植来自Clec12a−/−动物的微生物群足以加重野生型小鼠的结肠炎。Clec12a - / -小鼠已经扩大了啮齿Faecalibaculum rodentium,并且用f.r odenium治疗同样会加剧疾病。然而,当Clec12a - / -动物重新衍生为11个成员的群落时,它们对结肠炎的发展具有抗性,这强调了特定物种的作用。Clec12a - / -小鼠的结肠炎依赖于单核细胞,Clec12a - / -巨噬细胞和血清中的细胞因子和测序分析显示炎症增强,吞噬基因减少。啮齿弧菌特异性地与Clec12a结合,Clec12a - / -缺失的巨噬细胞吞噬啮齿弧菌的能力受损。因此,Clec12a有助于先天免疫监视机制,控制潜在有害共生体的扩张,同时限制炎症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


