The intestinal fungus Aspergillus tubingensis promotes polycystic ovary syndrome through a secondary metabolite
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Polycystic ovary syndrome (PCOS) affects 6%–10% of women of reproductive age and is known to be associated with disruptions in the gut bacteria. However, the role of the gut mycobiota in PCOS pathology remains unclear. Using culture-dependent and internal transcribed spacer 2 (ITS2)-sequencing methods, we discovered an enrichment of the gut-colonizable fungus Aspergillus tubingensis in 226 individuals, with or without PCOS, from 3 different geographical areas within China. Colonization of mice with A. tubingensis led to a PCOS-like phenotype due to inhibition of Aryl hydrocarbon receptor (AhR) signaling and reduced interleukin (IL)-22 secretion in intestinal group 3 innate lymphoid cells (ILC3s). By developing a strain-diversity-based-activity metabolite screening workflow, we identified secondary metabolite AT-C1 as an endogenous AhR antagonist and a key mediator of PCOS. Our findings demonstrate that an intestinal fungus and its secondary metabolite play a critical role in PCOS pathogenesis, offering a therapeutic strategy for improving the management of the disease.
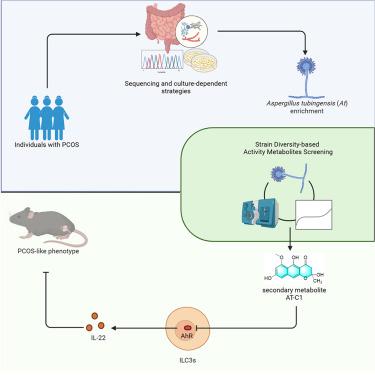
肠道真菌塔宾曲霉通过次级代谢物促进多囊卵巢综合征
多囊卵巢综合征(PCOS)影响6%-10%的育龄妇女,已知与肠道细菌的破坏有关。然而,肠道菌群在多囊卵巢综合征病理中的作用尚不清楚。利用培养依赖和内部转录间隔2 (ITS2)测序方法,我们在中国3个不同地理区域的226个有或没有PCOS的个体中发现了可肠道定植的真菌塔宾曲霉(Aspergillus tubingensis)。由于抑制芳烃受体(AhR)信号传导和减少肠道第3组先天淋巴样细胞(ILC3s)中白细胞介素(IL)-22的分泌,tubingensis定植小鼠导致pcos样表型。通过建立基于菌株多样性的活性代谢物筛选流程,我们确定了次生代谢物AT-C1是内源性AhR拮抗剂和PCOS的关键介质。我们的研究结果表明,肠道真菌及其次生代谢物在多囊卵巢综合征的发病机制中起着关键作用,为改善该病的治疗提供了一种治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
文献相关原料
公司名称
产品信息
索莱宝
Stool Genomic DNA Extraction Kit
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


