Chromosome mis-segregation triggers cell cycle arrest through a mechanosensitive nuclear envelope checkpoint
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
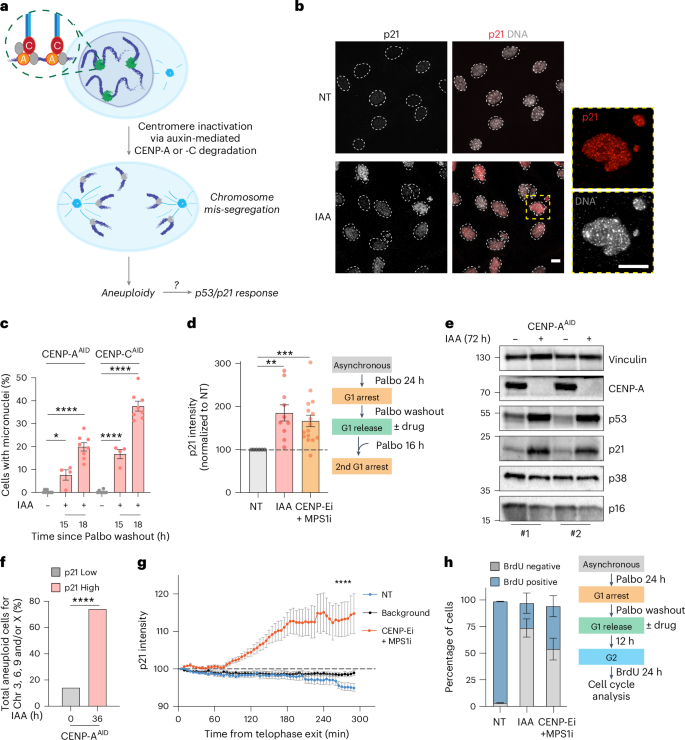
Errors during cell division lead to aneuploidy, which is associated with genomic instability and cell transformation. In response to aneuploidy, cells activate the tumour suppressor p53 to elicit a surveillance mechanism that halts proliferation and promotes senescence. The molecular sensors that trigger this checkpoint are unclear. Here, using a tunable system of chromosome mis-segregation, we show that mitotic errors trigger nuclear deformation, nuclear softening, and lamin and heterochromatin alterations, leading to rapid p53/p21 activation upon mitotic exit in response to changes in nuclear mechanics. We identify mTORC2 and ATR as nuclear deformation sensors upstream of p53/p21 activation. While triggering mitotic arrest, the chromosome mis-segregation-induced alterations of nuclear envelope mechanics provide a fitness advantage for aneuploid cells by promoting nuclear deformation resilience and enhancing pro-invasive capabilities. Collectively, this work identifies a nuclear mechanical checkpoint triggered by altered chromatin organization that probably plays a critical role in cellular transformation and cancer progression. Hervé, Scelfo et al. show that chromosome mis-segregation induces mTORC2- and ATR-mediated p53 activation through a mechanosensitive checkpoint at the nuclear envelope triggered by altered heterochromatin content and increased nuclear membrane tension.

染色体错分离通过机械敏感的核膜检查点触发细胞周期阻滞
细胞分裂过程中的错误导致非整倍体,这与基因组不稳定性和细胞转化有关。在对非整倍体的反应中,细胞激活肿瘤抑制因子p53,引发一种阻止增殖和促进衰老的监视机制。触发这个检查点的分子传感器尚不清楚。在这里,我们使用一个可调节的染色体错误分离系统,我们发现有丝分裂错误触发核变形,核软化,核层蛋白和异染色质改变,导致有丝分裂退出后快速激活p53/p21,以响应核力学的变化。我们发现mTORC2和ATR是p53/p21激活上游的核变形传感器。在触发有丝分裂阻滞的同时,染色体错分离诱导的核膜力学改变通过促进核变形弹性和增强前侵入能力,为非整倍体细胞提供了适应性优势。总的来说,这项工作确定了由染色质组织改变触发的核机械检查点,该检查点可能在细胞转化和癌症进展中起关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


