Combining Photochemical Oxyfunctionalization and Enzymatic Catalysis for the Synthesis of Chiral Pyrrolidines and Azepanes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Chiral heterocyclic alcohols and amines are frequently used building blocks in the synthesis of fine chemicals and pharmaceuticals. Herein, we report a one-pot photoenzymatic synthesis route for N-Boc-3-amino/hydroxy-pyrrolidine and N-Boc-4-amino/hydroxy-azepane with up to 90% conversions and >99% enantiomeric excess. The transformation combines a photochemical oxyfunctionalization favored for distal C–H positions with a stereoselective enzymatic transamination or carbonyl reduction step. Our study demonstrates a mild and operationally simple asymmetric synthesis workflow from easily available starting materials.
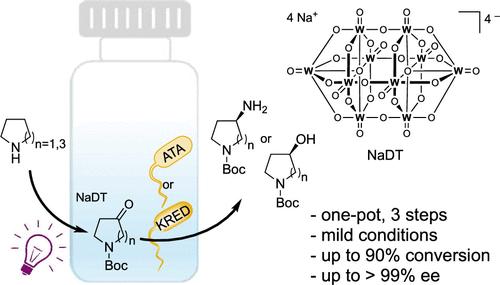
结合光化学氧化功能化和酶催化合成手性吡咯烷和氮杂烷
手性杂环醇和胺是精细化学品和药品合成中经常使用的原料。在此,我们报道了一锅光酶合成n - boc -3-氨基/羟基吡咯烷和n - boc -4-氨基/羟基氮杂烷的途径,转化率高达90%,对映体过量达99%。该转化结合了有利于远端C-H位置的光化学氧化官能化和立体选择性酶转氨化或羰基还原步骤。我们的研究展示了一个温和和操作简单的不对称合成工作流程,从容易获得的起始材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


