A distinct hypothalamus–habenula circuit governs risk preference
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
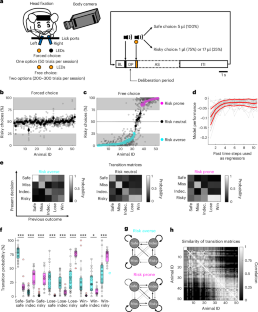
Appropriate risk evaluation is essential for survival in complex, uncertain environments. Confronted with choosing between certain (safe) and uncertain (risky) options, animals show strong preference for either option consistently across extended time periods. How such risk preference is encoded in the brain remains elusive. A candidate region is the lateral habenula (LHb), which is prominently involved in value-guided behavior. Here, using a balanced two-alternative choice task and longitudinal two-photon calcium imaging in mice, we identify risk-preference-selective activity in LHb neurons reflecting individual risk preference before action selection. By using whole-brain anatomical tracing, multi-fiber photometry and projection-specific and cell-type-specific optogenetics, we find glutamatergic LHb projections from the medial (MH) but not lateral (LH) hypothalamus providing behavior-relevant synaptic input before action selection. Optogenetic stimulation of MH→LHb axons evoked excitatory and inhibitory postsynaptic responses, whereas LH→LHb projections were excitatory. We thus reveal functionally distinct hypothalamus–habenula circuits for risk preference in habitual economic decision-making. Groos et al. show that lateral habenula activity reflects individual risk preference before action selection. This activity is modulated by behavior-relevant synaptic input from the medial hypothalamus capable of glutamate and GABA co-release.

一个独特的下丘脑-缰核回路控制着风险偏好
适当的风险评估对于在复杂、不确定的环境中生存至关重要。面对确定(安全)和不确定(风险)选项之间的选择,动物在很长一段时间内始终表现出强烈的偏好。这种风险偏好是如何在大脑中编码的仍然是一个谜。一个候选区域是外侧habenula (LHb),它在价值导向行为中起着重要作用。在这里,我们使用平衡的两种选择任务和小鼠纵向双光子钙成像,我们确定了LHb神经元的风险偏好选择活动,反映了个体在行动选择之前的风险偏好。通过全脑解剖追踪、多纤维光度测定以及投射特异性和细胞类型特异性光遗传学,我们发现来自内侧下丘脑(MH)而非外侧下丘脑(LH)的谷氨酸能LHb投射在动作选择之前提供了与行为相关的突触输入。光遗传刺激MH→LHb轴突引起兴奋性和抑制性突触后反应,而LH→LHb轴突则引起兴奋性反应。因此,我们揭示了在习惯性经济决策中风险偏好的功能不同的下丘脑-缰核回路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


