Toxic Effects of Butanol in the Plane of the Cell Membrane
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
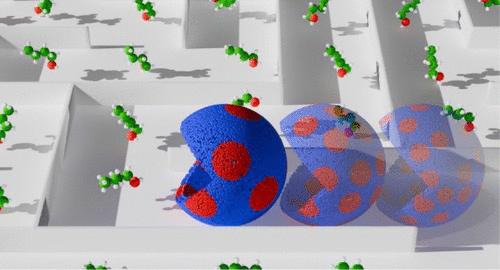
Solvent toxicity limits n-butanol fermentation titer, increasing the cost and energy consumption for subsequent separation processes and making biobased production more expensive and energy-intensive than petrochemical approaches. Amphiphilic solvents such as n-butanol partition into the cell membrane of fermenting microorganisms, thinning the transverse structure, and eventually causing a loss of membrane potential and cell death. In this work, we demonstrate the deleterious effects of n-butanol partitioning upon the lateral dimension of the membrane structure, called membrane domains or lipid rafts. Lipid rafts are regions of the cell membrane enriched with certain lipids, providing a reservoir of high melting temperature lipids and a platform for membrane protein partitioning and oligomerization. Neutron scattering experiments and molecular dynamics simulations revealed that n-butanol increased the size of the lipid domains in a model membrane system. The data showed that n-butanol partitions more into the disordered lipid regions than into the raft-like phase, leading to a differential thinning of these coexisting phases in the plane of the membrane and increasing the hydrophobic mismatch. The resulting increase in line tension at the interface favors domain coalescence to minimize the ratio of the interfacial length to domain area. A detailed computational investigation of the lipid domain interface identifies the boundary as a site of membrane disorder and thinning due to an accumulation of n-butanol. Solvent-induced changes to domain morphology and membrane instability at the domain interface are unrecognized modes of solvent-induced stress to fermenting microbes, representing targets for new solvent tolerance strategies to increase the n-butanol titer.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


