Manganese Galvanic Cells Intervene in Tumor Metabolism to Reinforce cGAS-STING Activation for Bidirectional Synergistic Hydrogen-Immunotherapy
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
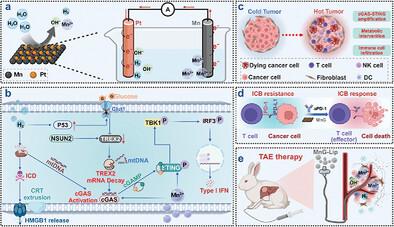
The cGAS-STING pathway is pivotal in initiating antitumor immunity. However, tumor metabolism, particularly glycolysis, negatively regulates the activation of the cGAS-STING pathway. Herein, Mn galvanic cells (MnG) are prepared via liquid-phase exfoliation and in situ galvanic replacement to modulate tumor metabolism, thereby enhancing cGAS-STING activation for bidirectional synergistic H2-immunotherapy. The obtained MnG can be etched by water, enabling efficient and sustained generation of H2 gas and Mn2+. MnG not only activated and amplified the cGAS-STING pathway through the sustained release of Mn2+ but also regulated tumor glucose metabolism to inhibit the expression of three prime repair exonuclease 2 (TREX2), thereby synergistically enhancing the activation of the cGAS-STING pathway. The injection of MnG into tumors resulted in a robust immune response, thereby providing favorable support for antitumor therapy. Consequently, the combination of MnG with immune checkpoint blockade therapy resulted in significant suppression of both primary tumors and distant tumors. Furthermore, the MnG-lipiodol dispersion exhibited remarkable efficacy in combination with transarterial embolization (TAE)-gas-immunotherapy in a rabbit orthotopic liver tumor model. The present study underscores the significance of employing a metal galvanic cell strategy for enhanced immunotherapy, thereby offering a novel approach for rational design of bioactive materials to augment immunotherapeutic effectiveness.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


