Modeling-Assisted Elucidation of the Organosolv Lignin Depolymerization: Lessons Learned from β-Ether Cleavage over Ni/C
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
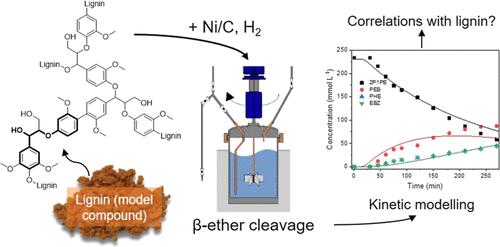
The complexity of lignin is a major challenge to overcome in order to develop a complete biorefinery concept for the biobased community. Therefore, the lignin model compound 2-phenoxy-1-phenylethanol was used to design lignin depolymerization. We proposed a two-step mechanism involving predehydrogenation at the Cα-position, removal of the OH group, and subsequent cleavage of the β-O-4 bond at the Cβ-position into phenol and ethylbenzene. The study was supported by density functional theory and kinetic modeling to evaluate the activation barriers for the cleavage of the β-O-4 bond in the dimeric lignin compound. The activation energies for predehydrogenation and cleavage at the Cβ-position of phenethoxybenzene were predicted to be 71 kJ mol–1 and 9 kJ mol–1, respectively, suggesting that the predehydrogenation is beneficial for the cleavage of the β-O-4 bond as it lowers the activation energy. Additionally, the removal of the OH group at the Cα-position increased the reaction rate constant for the β-O-4 bond cleavage to 0.68 min–1. By comparing lignin depolymerization and the cleavage of the β-O-4 bond in the dimeric lignin compound, the study provided mechanistic insights and suggested process- and structure-dependent correlations. Similarities were found in the process mechanism of aliphatic OH group removal and cleavage at the Cβ-position, while the temperature increase contributed more to the enhanced cleavage of the β-O-4 bond in the lignin model compound compared to the lignin macromolecule. On the other hand, the reaction conditions affected the structural characteristics of the products after lignin depolymerization, especially the molecular weight and functionality of the oligomeric fragments. We have found that using a lignin model component is beneficial for fundamental research, but correlating the results with the real lignin sample is essential to improve the potential of lignin in the biorefinery concept.
模拟辅助阐释有机溶剂木质素解聚:Ni/C上β-醚裂解的经验教训
为了开发一个完整的生物基生物炼制概念,木质素的复杂性是一个需要克服的主要挑战。因此,采用木质素模型化合物2-苯氧基-1-苯乙醇设计木质素解聚。我们提出了一个两步机制,即在c α-位置预脱氢,去除OH基团,然后在c β-位置裂解β-O-4键,生成苯酚和乙苯。利用密度泛函理论和动力学模型对二聚木质素化合物中β-O-4键断裂的激活障碍进行了研究。预测苯乙氧基苯c - β位预脱氢和裂解的活化能分别为71 kJ mol-1和9 kJ mol-1,表明预脱氢降低了活化能,有利于β-O-4键的裂解。此外,去除c α-位置的OH基团使β-O-4键的裂解反应速率常数提高到0.68 min-1。通过比较木质素解聚和二聚木质素化合物中β-O-4键的断裂,该研究提供了机理见解,并提出了过程和结构依赖的相关性。与木质素大分子相比,木质素模型化合物中脂肪族羟基的去除和c - β位置的裂解过程机制相似,而温度的升高更有助于木质素模型化合物中β-O-4键的裂解增强。另一方面,反应条件影响木质素解聚后产物的结构特征,特别是低聚片段的分子量和功能。我们发现,使用木质素模型组分有利于基础研究,但将结果与实际木质素样品相关联对于提高木质素在生物炼制概念中的潜力至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


