Inducing Cu Charge Redistribution by Modulating Proximity with Zr(OH)4 for Selective Synthesis of Imines and Secondary Amines with Stoichiometric Benzyl Alcohol and Nitrobenzene
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
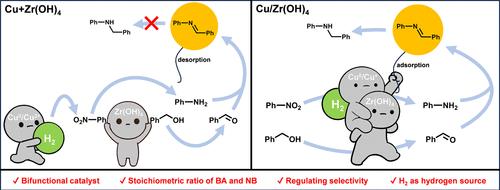
The one-pot synthesis of amines from benzyl alcohol (BA) and nitrobenzene (NB) represents a step-economic method. However, reported works typically require more than 3 equiv of BA to complete the transfer hydrogenation of NB, and few studies can achieve selective synthesis of imines and secondary amines. In our previous work, Zr(OH)4 demonstrated both hydrogenation and dehydrogenation capabilities. Building on this, another component Cu was introduced to enhance its catalytic performance for catalyzing coupling reaction between stoichiometric BA and NB under a H2 atmosphere. The physical hybrid catalyst Cu + Zr(OH)4 selectively produced imines, while the supported catalyst Cu/Zr(OH)4 yielded secondary amines. Characterization and mechanism experiments revealed that modulating the proximity between Cu and Zr(OH)4 leads to (1) different adsorption abilities of the catalyst for N-benzylideneaniline (NBA) and (2) interactions between Zr(OH)4 and Cu in close contact, which stabilized the electronic structure of Cu forming more Cu+/Cu0 ion pairs with strong H2 activation ability. This work presents a catalyst design strategy and offers an approach for the selective preparation of N-benzylideneaniline and N-benzylaniline.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


