AuCu Nanodendrite for Enhancing Electrocatalytic Nitrate Reduction Applications via Two-stage Microfluidic Fabrication Strategy
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The electrocatalytic nitrate reduction reaction (NitrRR) has attracted great attention in clean ammonia production, but it has unsatisfactory selectivity and sluggish dynamics, owing to the complex eight-electron transfer process. While dendritic AuCu alloy is anticipated to offer competitive performance, significant challenges remain in terms of insufficient structural regulation and an unelucidated reaction enhancement mechanism because of the complexity involved in its preparation. To address these issues, we have developed a two-stage microfluidic platform that facilitates the stable fabrication and controllable regulation of AuCu nano dendrites (NDs). Notably, the Cu content in the resultant AuCu NDs reaches an impressive 35.34 At%, surpassing traditional liquid-phase reduction limitations. Furthermore, the dendrite structure has been thoroughly validated, revealing a clear structure–activity relationship. By employing precise manipulation, we have determined the optimal composition of AuCu NDs, achieving a remarkable ammonia yield of 21.93 mg h–1 cm–2 and a faradic efficiency of 93.30%. Additionally, DFT calculations further elucidate the performance enhancement mechanism, showing that Au3Cu1 sites in the AuCu NDs significantly reduce the energy barrier (0.28 eV) of the rate-determining step (RDS: *NO → *HNO), while excessive Cu deposition has an adverse effect. Our work contributes innovative guidance for the design and controllable fabrication of high-performance electrocatalysts.
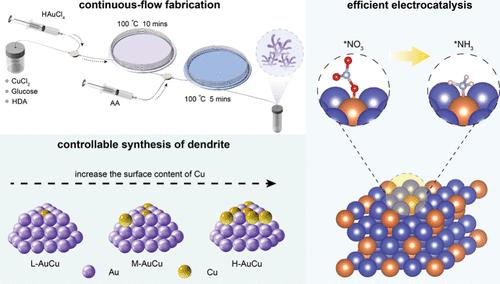
两级微流控制备技术增强硝酸还原电催化应用的AuCu纳米枝晶
电催化硝酸还原反应(NitrRR)在清洁氨生产中备受关注,但由于其复杂的八电子传递过程,选择性不理想,动力学迟缓。虽然树枝状AuCu合金有望提供具有竞争力的性能,但由于其制备过程的复杂性,其结构调控不足和反应增强机制尚不明确,因此仍然存在重大挑战。为了解决这些问题,我们开发了一个两阶段的微流控平台,促进了AuCu纳米枝晶(NDs)的稳定制造和可控调节。值得注意的是,所得的AuCu nd中的Cu含量达到了令人印象深刻的35.34 At%,超过了传统的液相还原限制。此外,树突结构已被彻底验证,揭示了明确的构效关系。通过精确的操作,我们确定了AuCu NDs的最佳组成,获得了21.93 mg h-1 cm-2的氨收率和93.30%的效率。此外,DFT计算进一步阐明了性能增强机制,表明Au3Cu1位点显著降低了速率决定步骤(RDS: *NO→*HNO)的能垒(0.28 eV),而过量的Cu沉积则有不利影响。我们的工作为高性能电催化剂的设计和可控制造提供了创新指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
文献相关原料
公司名称
产品信息
阿拉丁
Chloroauric acid
阿拉丁
Copper chloride dihydrate
阿拉丁
Potassium hydroxide
阿拉丁
Potassium nitrate
阿拉丁
Sodium hydroxide
阿拉丁
Salicylic acid
阿拉丁
Sodium nitroprusside
阿拉丁
Sulfanilamide
阿拉丁
N-(1-naphthyl) ethylenediamine dihydrochloride
阿拉丁
Phosphoric acid
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


