Dynamic Catalysis Multiscale Simulations for Nonoxidative Coupling of Methane Using Light and Heat
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
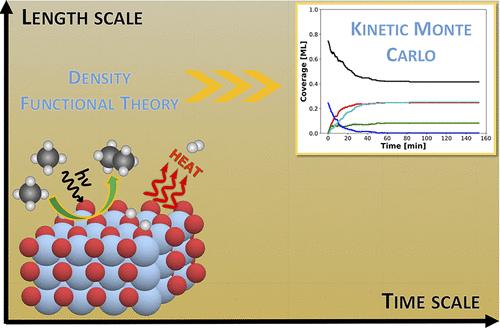
Methane (CH4) activation and conversion under mild reaction conditions are a great challenge for the chemical industry. Photocatalysis is attractive for activating inert C–H bonds of CH4 at room temperature. Specifically, photocatalytic nonoxidative coupling of CH4 (NOCM) is a promising process to produce ethane (C2-hydrocarbon) and H2. Different oxide-based photocatalysts have been used for room-temperature NOCM, and TiO2 is a potential photocatalyst with a bandgap that can capture photons in the UV region. However, a fundamental understanding of the NOCM mechanism on TiO2 is still missing. Herein, we apply multiscale modeling, combining density functional theory (DFT) calculations with kinetic Monte Carlo (kMC) simulations to investigate the photocatalytic NOCM on a rutile TiO2(110) surface. DFT calculations revealed that the photogenerated holes mediate the homolytic activation of CH4 via the formation of methyl radicals with an activation barrier that is 70% lower than that of the conventional thermocatalytic route. The generated methyl radicals further recombine to form ethane. The detailed reaction pathway energetics investigated with DFT-based kMC simulations revealed that ethane can be formed at 315.15 K, but the dissociated hydrogens poison the catalyst surface. Further thermocatalytic simulations revealed that increasing the temperature by thermal heating (ca. 690.15 K) facilitated H2 formation and catalyst regeneration. Importantly, we demonstrate how photo- and thermocatalytic modes can be combined, facilitating NOCM on TiO2 and a route to enable dynamic catalysis simulations through multiscale modeling, opening alternative avenues in computational catalyst discovery.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


