Visible light-driven selective oxidation of multiple organic matters by photocatalysis of BiOX (X = I, Br): New insights into the role of oxygen vacancy generation and electron gain/loss properties of matter
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Realizing better photocatalytic activity of materials by various strategies has always been a hot topic in the field of environmental catalysis. However, the complex heterogeneous composition and preparation process of catalysts undoubtedly limit their potential for practical pollution control. Therefore, clarifying the selective photocatalytic mechanism of pristine semiconductor oxide and the crucial relationship between catalysts structures and physical–chemical characteristics of target pollutants become more necessary. In our study, based on the combination of density functional theory (DFT) calculation and characterizations, the differences in photocatalytic degradation behavior and intrinsic mechanisms of common organic pollutants including ciprofloxacin (CIP), tetracycline (TC), Rhodamine B (RhB) and bisphenol-A (BPA) over the pristine BiOBr and BiOI were investigated, thereby clarifying the crucial relationship and synergistic effects between photocatalysts microstructure and pollutants characteristics. Under the same reaction conditions, 10 %-BiOBr (BiOBr obtained from the hydrothermal treatment in a 10 % ethylene glycol solution environment) exhibited better degradation performance of CIP (73 %), TC (67 %) and RhB (95 %) than that of BPA (28 %). While 10 %-BiOI (BiOI obtained from the hydrothermal treatment in a 10 % ethylene glycol solution environment) exhibited better degradation performance of BPA (88 %) than those of CIP (27 %), TC (60 %) and RhB (41 %). The obvious selective degradation difference of various target pollutants over the 10 %-BiOBr and 10 %-BiOI could be ascribed as follows: (i) Under the same synthesis condition, the differences in Bi![]() O bond lengths due to the halogen layers resulted in BiOI being more susceptible to generate oxygen vacancy than BiOBr, thereby boosting the non-radicalpathway degradation of electron-rich pollutants. (ii) The generation of radicals and non-radicals during photocatalysis were directly affected by the electronic characteristic discrepancies of the pollutants, thereby changing the main degradation pathways of organic matters. Results indicated that the electron-deficient pollutants including CIP, TC and RhB tended to be degraded by radical pathway. On the contrary electron-rich pollutant BPA was more susceptible to degradation via direct electrons transfer to oxygen vacancies of 10 %-BiOI. Our study supplied new insight into adopting pristine photocatalysts based on the difference of oxygen vacancy for selectively degrading various pollutants with different donating/losing electron ability, the corresponding mechanisms and degradation pathway could provide theoretical reference for practical application of pristine photocatalysts.
O bond lengths due to the halogen layers resulted in BiOI being more susceptible to generate oxygen vacancy than BiOBr, thereby boosting the non-radicalpathway degradation of electron-rich pollutants. (ii) The generation of radicals and non-radicals during photocatalysis were directly affected by the electronic characteristic discrepancies of the pollutants, thereby changing the main degradation pathways of organic matters. Results indicated that the electron-deficient pollutants including CIP, TC and RhB tended to be degraded by radical pathway. On the contrary electron-rich pollutant BPA was more susceptible to degradation via direct electrons transfer to oxygen vacancies of 10 %-BiOI. Our study supplied new insight into adopting pristine photocatalysts based on the difference of oxygen vacancy for selectively degrading various pollutants with different donating/losing electron ability, the corresponding mechanisms and degradation pathway could provide theoretical reference for practical application of pristine photocatalysts.
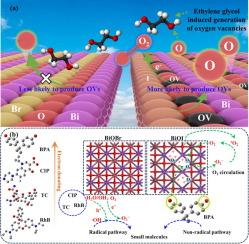
BiOX (X = I, Br)光催化下多种有机物的可见光选择性氧化:氧空位生成和物质电子增益/损失特性的新见解
通过各种策略实现材料更好的光催化活性一直是环境催化领域的研究热点。然而,催化剂复杂的非均相组成和制备工艺无疑限制了其在实际污染控制中的潜力。因此,澄清原始半导体氧化物的选择性光催化机理以及催化剂结构与目标污染物的物理化学特性之间的重要关系变得更加必要。本研究基于密度泛函理论(DFT)计算和表征相结合的方法,研究了环丙沙星(CIP)、四环素(TC)、罗丹明B (RhB)和双酚a (BPA)等常见有机污染物在原始BiOBr和biobi上的光催化降解行为差异及其内在机制,从而阐明了光催化剂微观结构与污染物特性之间的重要关系和协同效应。在相同的反应条件下,10 %-BiOBr(在10 %乙二醇溶液环境中水热处理得到的BiOBr)对CIP(73 %)、TC(67 %)和RhB(95 %)的降解性能优于BPA(28 %)。而10 %-BiOI(在10 %乙二醇溶液环境中水热处理得到的BiOI)对BPA的降解性能(88 %)优于CIP(27 %)、TC(60 %)和RhB(41 %)。各种目标污染物在10 %-BiOBr和10 %-BiOI上选择性降解的明显差异可以解释为:(i)在相同的合成条件下,由于卤素层导致的BiO键长度的差异,导致在相同的催化剂制备条件下,BiOI比BiOBr更容易产生氧空位,从而促进了非自由基对富电子污染物的降解。(ii)光催化过程中自由基和非自由基的生成直接受到污染物电子特性差异的影响,从而改变了有机物的主要降解途径。结果表明,CIP、TC和RhB等缺电子污染物倾向于通过自由基途径降解。相反,富含电子的污染物BPA更容易通过直接电子转移到氧空位10 %-BiOI而被降解。本研究为采用基于氧空位差异的原始光催化剂选择性降解各种给失电子能力不同的污染物提供了新的思路,相应的机理和降解途径可为原始光催化剂的实际应用提供理论参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


