Targeting N-Myc in neuroblastoma with selective Aurora kinase A degraders
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The N-Myc transcription factor, encoded by MYCN, is a mechanistically validated, yet challenging, target for neuroblastoma (NB) therapy development. In normal neuronal progenitors, N-Myc undergoes rapid degradation, while, in MYCN-amplified NB cells, Aurora kinase A (Aurora-A) binds to and stabilizes N-Myc, resulting in elevated protein levels. Here, we demonstrate that targeted protein degradation of Aurora-A decreases N-Myc levels. A potent Aurora-A degrader, HLB-0532259 (compound 4), was developed from an Aurora-A-binding ligand that engages the Aurora-A/N-Myc complex. HLB-0532259 promotes the degradation of Aurora-A, which elicits concomitant N-Myc degradation, with nanomolar potency and excellent selectivity. HLB-0532259 surpasses the cellular efficacy of established allosteric Aurora-A inhibitors, exhibits favorable pharmacokinetic properties, and elicits tumor reduction in a murine xenograft NB model. This study broadly delineates a strategy for targeting “undruggable” proteins that are reliant on accessory proteins for cellular stabilization.
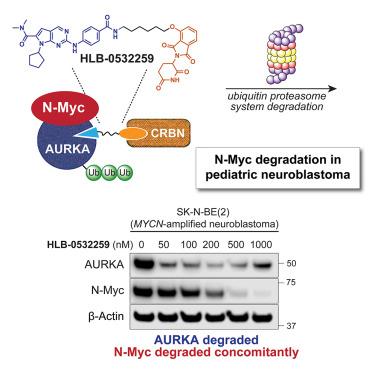

选择性极光激酶A降解物靶向神经母细胞瘤N-Myc
由MYCN编码的N-Myc转录因子是神经母细胞瘤(NB)治疗发展的一个机制验证但具有挑战性的靶点。在正常的神经元祖细胞中,N-Myc经历快速降解,而在mycn扩增的NB细胞中,极光激酶A (Aurora-A)结合并稳定N-Myc,导致蛋白水平升高。在这里,我们证明了Aurora-A的靶向蛋白降解降低了N-Myc水平。一种有效的Aurora-A降解剂HLB-0532259(化合物4)是由Aurora-A结合配体与Aurora-A/N-Myc复合物结合而成的。HLB-0532259促进极光a的降解,同时引起N-Myc的降解,具有纳米摩尔的效力和良好的选择性。HLB-0532259优于已建立的变构性Aurora-A抑制剂的细胞功效,表现出良好的药代动力学特性,并在小鼠异种移植物NB模型中诱导肿瘤减少。这项研究广泛地描述了一种靶向“不可药物”蛋白质的策略,这些蛋白质依赖于辅助蛋白质来实现细胞稳定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


