Deposition and decomposition of electrolyte solutes caused by N-doped porous carbon: a kinetic study of ion migration
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Nitrogen-doped porous carbon has been widely used in electrochemical energy storage, particularly in electrochemical double-layer capacitors (EDLCs), where it demonstrates excellent capacitance performance. However, the impact of nitrogen doping on the migration of electrolyte ions and the rapid decay of electrochemical performance at high temperatures has been rarely reported. In this work, a series of advanced characterizations, such as electrochemical quartz crystal microbalance (EQCM), time-of-flight secondary ion mass spectrometry (TOF-SIMS), and synchrotron radiation-based X-ray absorption near edge structure (XANES), were employed to analyze ion migration, deposition, and decomposition. Combining first-principles calculations, it was proved that the high-energy state lone pair electrons of amine and pyrrole-N trigger the continuous deposition/decomposition of anions BF4-, and prevent the deposition of cations SBP+. The deposited and decomposed cations further formed a passivation interface, which hindered ion migration. This interface led to dramatic fluctuations in electrode mass changes during charging and discharging, and reduced the total amount of ion migration. This work provides a novel kinetic study of ion migration within the electrochemical interface of nitrogen-doped porous carbon, which could contribute to enhancing the specific capacitance and cycle life of supercapacitors.
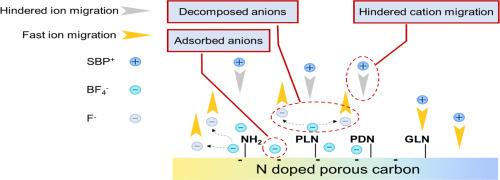
掺n多孔碳引起的电解质溶质沉积和分解:离子迁移的动力学研究
氮掺杂多孔碳在电化学储能领域有着广泛的应用,特别是在电化学双层电容器(edlc)中表现出优异的电容性能。然而,氮掺杂对电解质离子迁移和高温下电化学性能快速衰减的影响却鲜有报道。在这项工作中,采用电化学石英晶体微天平(EQCM)、飞行时间二次离子质谱(TOF-SIMS)和基于同步辐射的x射线吸收近边结构(XANES)等一系列先进的表征方法来分析离子的迁移、沉积和分解。结合第一性原理计算,证明了胺和吡罗- n的高能态孤对电子触发阴离子BF4-的连续沉积/分解,并阻止阳离子SBP+的沉积。沉积分解的阳离子进一步形成钝化界面,阻碍了离子的迁移。该界面导致了充放电过程中电极质量变化的剧烈波动,减少了离子迁移总量。本研究为氮掺杂多孔碳的电化学界面内离子迁移提供了一种新的动力学研究,有助于提高超级电容器的比电容和循环寿命。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


