Structural basis for the inhibition of PRC2 by active transcription histone posttranslational modifications
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
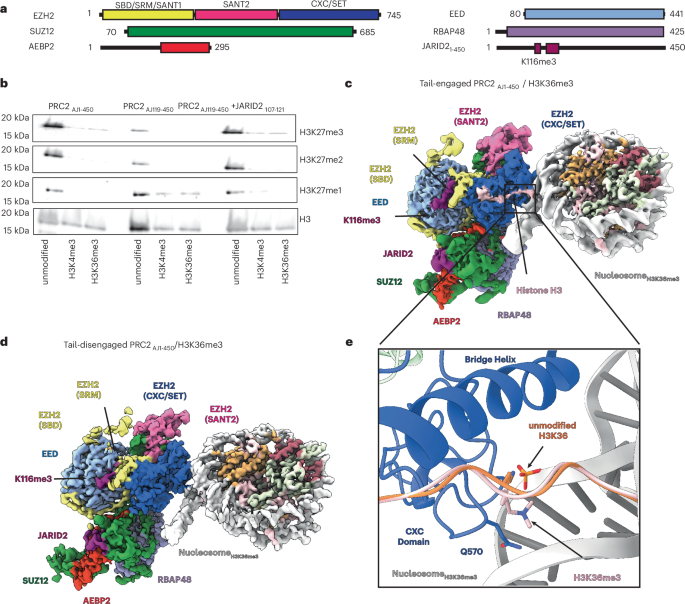
Polycomb repressive complex 2 (PRC2) trimethylates histone H3 on K27 (H3K27me3) leading to gene silencing that is essential for embryonic development and maintenance of cell identity. PRC2 is regulated by protein cofactors and their crosstalk with histone modifications. Trimethylated histone H3 on K4 (H3K4me3) and K36 (H3K36me3) localize to sites of active transcription and inhibit PRC2 activity through unknown mechanisms. Using cryo-electron microscopy, we reveal that histone H3 tails containing H3K36me3 engage poorly with PRC2 and preclude its effective interaction with chromatin, while H3K4me3 binds to the allosteric site in the EED subunit, acting as an antagonist that competes with activators required for spreading of the H3K27me3 repressive mark. Thus, the location of the H3K4me3 and H3K36me3 modifications along the H3 tail allows them to target two requirements for efficient trimethylation of H3K27 by PRC2. We further show that the JARID2 cofactor modulates PRC2 activity in the presence of these histone modifications. Structures reveal that histone H3K36me3 and H3K4me3 modifications reduce Polycomb repressive complex 2 (PRC2) function through the inhibition of histone tail engagement and antagonistic binding to the allosteric site, two important requirements for the efficient trimethylation of H3K27 by PRC2.

活性转录组蛋白翻译后修饰抑制PRC2的结构基础
多梳抑制复合体2 (PRC2)三甲基化K27上的组蛋白H3 (H3K27me3),导致基因沉默,这对胚胎发育和维持细胞身份至关重要。PRC2受蛋白辅助因子及其与组蛋白修饰的串扰调控。K4 (H3K4me3)和K36 (H3K36me3)上的三甲基化组蛋白H3定位于活性转录位点,并通过未知的机制抑制PRC2活性。通过低温电子显微镜,我们发现含有H3K36me3的组蛋白H3尾部与PRC2的结合很差,阻碍了其与染色质的有效相互作用,而H3K4me3与EED亚基的变抗位点结合,作为拮抗剂与H3K27me3抑制标记扩散所需的激活剂竞争。因此,H3K4me3和H3K36me3修饰在H3尾部的位置使它们能够针对PRC2对H3K27进行有效三甲基化的两个要求。我们进一步表明,在这些组蛋白修饰存在的情况下,JARID2辅因子调节PRC2活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


