Synthesis and discovery of simplified pleurotin analogs bearing tricyclic core as novel thioredoxin reductase inhibitors
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
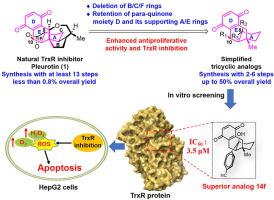
Pleurotin (1) is a benzoquinone meroterpenoid known for its wide-spectrum antitumor and antibiotic activities, notably acting as natural inhibitors of the thioredoxin reductase (TrxR). Pleurotin (1) has been chemically synthesized, but only in milligram quantities through at least 13 longest linear steps with 0.8 % overall yield due to its complex structure such as fused hexacyclic core with 8 contiguous stereocenters. Therefore, structural simplification strategy is applied to pleurotin natural products for their structure-activity relationship (SAR) study and further therapeutics development. Herein, we judiciously designed pleurotin analogs of tricyclic A/D/E ring core, retaining the putative pharmacophore of para-quinone moiety D and its supportive A and E rings. Thus 16 simplified analogs of pleurotin bearing tricyclic A/D/E core were readily synthesized in only 2 to 6 steps with up to 50 % overall yield from commercially available materials. Significantly, the best analog 14f with benzonitrile substituent exhibited more potent TrxR inhibitory activity with an IC50 of 3.5 μM than the positive control micheliolide (IC50 = 6.23 μM). Furthermore, the mechanism study revealed that compound 14f could induce apoptosis of tumor cells by inducing ROS generation and inhibiting TrxR activities. Our study for the first time showed that the tricyclic A/D/E ring scaffold from the natural product pleurotin (1) with proper substitution can maintain or even improve the TrxR inhibitory and antiproliferative activities, with high synthetic accessibility, affording natural product-derived lead compounds for the further development of TrxR inhibitors as anti-tumor therapeutics.

新型硫氧还蛋白还原酶抑制剂三环核心简化胸膜蛋白类似物的合成与发现
Pleurotin (1) 是一种苯醌类蛇床子素,具有广泛的抗肿瘤和抗生素活性,尤其是作为硫代氧化还原酶 (TrxR) 的天然抑制剂。Pleurotin (1) 已被化学合成,但由于其结构复杂,如具有 8 个连续立体中心的融合六环核心,因此至少需要 13 个最长的线性步骤,总产率仅为 0.8%。因此,结构简化策略被应用于褶皱素天然产物的结构-活性关系(SAR)研究和进一步的治疗开发。在此,我们审慎地设计了以三环 A/D/E 环为核心的褶皱素类似物,保留了对醌分子 D 及其支持性 A 环和 E 环的推定药效源。因此,只需 2 到 6 个步骤,就能从市场上买到的材料中轻松合成出 16 种具有三环 A/D/E 核心的褶皱素简化类似物,总产率高达 50%。值得注意的是,带有苯甲腈取代基的最佳类似物 14f 表现出更强的 TrxR 抑制活性,其 IC50 为 3.5 μM,高于阳性对照 micheliolide(IC50 = 6.23 μM)。此外,机理研究还发现,化合物 14f 可通过诱导 ROS 生成和抑制 TrxR 活性来诱导肿瘤细胞凋亡。我们的研究首次表明,天然产物pleurotin(1)的三环A/D/E环支架经适当取代后,可保持甚至提高TrxR抑制和抗增殖活性,且具有较高的合成可及性,为进一步开发TrxR抑制剂作为抗肿瘤治疗药物提供了天然产物衍生的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


