Design, synthesis and biological evaluation of bisnoralcohol derivatives as novel IRF4 inhibitors for the treatment of multiple myeloma
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Interferon regulatory factor 4 (IRF4) is specifically overexpressed in multiple myeloma (MM) and mediates MM progression and survival, making it an emerging target for MM treatment. However, no chemical entity with a defined structure capable of directly binding to and inhibiting IRF4 has been reported. We screened our small library of steroid analogs and identified bisnoralcohol (BA) derivative 18 as a novel hit compound capable of inhibiting IRF4, with an IC50 of 13.46 μM. Based on 18, a series of BA derivatives was synthesized and evaluated for their inhibitory effects on IRF4 and antiproliferative activities against MM cell lines. Among these compounds, 41 (SH514) exhibited the highest potency, with an IC50 value of 2.63 μM for inhibiting IRF4, and IC50 values of 0.08 μM and 0.11 μM for inhibiting the proliferation of IRF4-high-expressing NCI–H929 and MM.1R MM cells, respectively. SH514 can bind to the IRF4-DBD domain with a KD of 1.28 μM. SH514 selectively and potently inhibits IRF4-high-expressing MM cells over IRF4-low-expressing MM cells. Mechanistic studies demonstrated that SH514 suppresses the downstream genes of IRF4, including CCNC, CANX, E2F5, CMYC, HK2, and Blimp1, and inhibited the expression of cell cycle-related proteins CDC2, Cyclin B1, Cyclin D1, Cyclin E1, and CMYC in MM cells. In vivo, SH514 effectively inhibited the proliferation of MM tumors, showing much better antitumor efficacy than the clinical drug lenalidomide, and exhibited no significant toxicity. Thus, these IRF4 inhibitors could serve as promising leads for the development of novel anti-multiple myeloma agents.
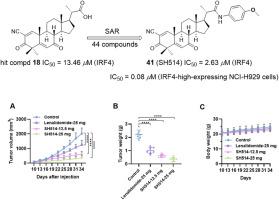

双诺醇衍生物作为新型IRF4抑制剂治疗多发性骨髓瘤的设计、合成和生物学评价
干扰素调节因子 4(IRF4)在多发性骨髓瘤(MM)中特异性过表达,并介导 MM 的进展和存活,使其成为治疗 MM 的新靶点。然而,目前还没有关于具有明确结构、能够直接与 IRF4 结合并抑制 IRF4 的化学实体的报道。我们筛选了我们的小型类固醇类似物文库,发现双花椒醇(BA)衍生物 18 是一种能够抑制 IRF4 的新型命中化合物,其 IC50 为 13.46 μM。以 18 为基础,合成了一系列 BA 衍生物,并评估了它们对 IRF4 的抑制作用以及对 MM 细胞株的抗增殖活性。在这些化合物中,41(SH514)表现出最高的效力,抑制 IRF4 的 IC50 值为 2.63 μM,抑制 IRF4 高表达的 NCI-H929 和 MM.1R MM 细胞增殖的 IC50 值分别为 0.08 μM 和 0.11 μM。SH514 与 IRF4-DBD 结构域的结合 KD 为 1.28 μM。与 IRF4 低表达的 MM 细胞相比,SH514 能选择性地有效抑制 IRF4 高表达的 MM 细胞。机理研究表明,SH514抑制了IRF4的下游基因,包括CCNC、CANX、E2F5、CMYC、HK2和Blimp1,并抑制了MM细胞中细胞周期相关蛋白CDC2、Cyclin B1、Cyclin D1、Cyclin E1和CMYC的表达。在体内,SH514能有效抑制MM肿瘤的增殖,其抗肿瘤疗效远优于来那度胺,且无明显毒性。因此,这些IRF4抑制剂有望成为开发新型抗多发性骨髓瘤药物的线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


