Development of molecular Trojan horses targeting New Delhi metallo-β-lactamase-1 for the restoration of meropenem susceptibility in drug-resistant bacteria
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The emergence of New Delhi metallo-β-lactamase-1 (NDM-1) poses a significant threat to the clinical application of antibiotics, as it possesses the ability to hydrolyze nearly all β-lactam antibiotics. Regrettably, there are currently no clinical drugs targeting NDM-1, making it imperative to develop highly potent and minimally toxic NDM-1 inhibitors. Herein, a series of molecular Trojan horses targeting NDM-1 were synthesized by introducing ebselen into 7-aminocephalosporanic acid derivatives via a C–Se bond. Representative compound 18b exhibited potent inhibitory activity against NDM-1, with an IC50 value of 7.03 μM, and combining with meropenem (Mem) decreased the minimum inhibitory concentration (MIC) of Mem by 4-32-fold in NDM-1 expressing bacteria. Mechanistically, 18b released the ebselen moiety at the active site of NDM-1, forming a Se–S bond with Cys208 to achieve targeted drug delivery of ebselen. Importantly, 18b demonstrated potent inhibition of resistant bacterial growth and replication in mice when administered in combination with Mem. These results suggest that 18b is a promising candidate for treating infections caused by resistant bacteria expressing NDM-1.
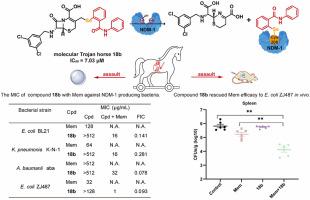

靶向新德里金属-β-内酰胺酶-1的分子特洛伊木马的开发,用于恢复耐药菌对美罗培南的敏感性
新德里金属β-内酰胺酶1 (New Delhi metallic -β-lactamase-1, NDM-1)的出现对抗生素的临床应用构成了重大威胁,因为它具有水解几乎所有β-内酰胺类抗生素的能力。遗憾的是,目前临床上还没有针对NDM-1的药物,因此开发高效、低毒的NDM-1抑制剂势在必行。本文通过C-Se键将艾布selen引入到7-氨基头孢菌酸衍生物中,合成了一系列靶向NDM-1的分子特洛伊木马。具有代表性的化合物18b对NDM-1表现出较强的抑制活性,IC50值为7.03 μM,与美罗培南(Mem)联用可使Mem在NDM-1表达菌中的最小抑制浓度(MIC)降低4 ~ 32倍。在机制上,18b在NDM-1的活性位点释放ebselen部分,与Cys208形成Se-S键,实现ebselen的靶向给药。重要的是,当与Mem联合使用时,18b在小鼠中显示出对耐药细菌生长和复制的有效抑制。这些结果表明,18b是治疗由表达NDM-1的耐药菌引起的感染的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


