Creating rich closed nanopores in anthracite-derived soft carbon enables greatly-enhanced sodium-ion storage in the low-working-voltage region
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
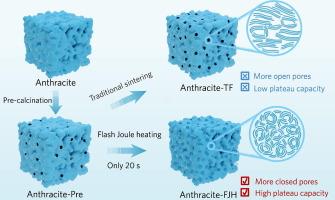
Soft carbon, characterized by its abundant reserves, low cost, and high carbon yield, should have been an important choice for anode materials in sodium-ion batteries (SIBs), similar to hard carbon. However, traditional high-temperature synthesis tends to graphitize soft carbon, which is extremely detrimental to the adsorption and intercalation of Na+ ions. This raises a highly challenging scientific question: whether it is possible to suppress the graphitization of soft carbon and transform it towards closed nanopores favorable for Na+ storage during high-temperature treatment. Herein, we introduce flash Joule heating (FJH) technology to treat anthracite with rapid heating and quenching, obtaining metastable soft carbon (anthracite-FJH) containing a large number of short-range ordered graphitic microdomains and their assembled closed nanopores. Benefiting from the abundant closed nanopores, this kinetically-controlled soft carbon exhibits a significantly enhanced Na+ adsorption and pore-filling capacity of 309 mAh g−1 at 0.1 C, dominantly contributed by the low-voltage plateau. Moreover, a reversible specific capacity of 256.2 mAh g−1 is maintained at 0.5 C with a capacity retention of 93.2 % after 200 cycles. We experimentally and theoretically explicitly disclose the “carbon microstructure regulation-Na+ storage mechanism” relationship. This work paves the way for the disruptive synthesis of high-capacity soft carbon SIB anodes based on anthracite
在无烟煤衍生的软碳中创建丰富的封闭纳米孔,可以在低工作电压区域大大增强钠离子的存储
软碳具有储量丰富、成本低、产碳率高等特点,与硬碳类似,应成为钠离子电池负极材料的重要选择。然而,传统的高温合成往往使软碳石墨化,这对Na+离子的吸附和插层极为不利。这就提出了一个极具挑战性的科学问题:是否有可能在高温处理过程中抑制软碳的石墨化,并将其转变为有利于Na+储存的封闭纳米孔。本文采用闪现焦耳加热(FJH)技术对无烟煤进行快速加热淬火处理,得到含有大量短程有序石墨微畴及其组装的封闭纳米孔的亚稳软碳(FJH)。得益于丰富的封闭纳米孔,该动力学控制软碳在0.1 C时表现出309 mAh g−1的Na+吸附和孔隙填充能力,这主要是由低压平台贡献的。此外,在0.5 ℃下可保持256.2 mAh g−1的可逆比容量,200次循环后容量保持率为93.2 %。从实验和理论上明确揭示了“碳微观结构调控- na +储存机制”的关系。这项工作为基于无烟煤的高容量软碳SIB阳极的破坏性合成铺平了道路
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


