Neoadjuvant chemoradiation with or without PD-1 blockade in locally advanced rectal cancer: a randomized phase 2 trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Radiotherapy displays unique antitumor synergism with immune checkpoint inhibitors, which is indicated by high pathological complete response (pCR) rates from single-arm trials of locally advanced rectal cancer (LARC). Here we test the efficacy and safety of the radiation–immune checkpoint inhibitor combination in patients with LARC in a phase 2, randomized trial conducted in eight major colorectal cancer centers in Beijing. In total, 186 eligible all-comer (proficient mismatch repair and deficient mismatch repair) participants were enrolled. The patients were randomly assigned to receive neoadjuvant chemoradiation + concurrent/sequential PD-1 blockade (experiment groups A/B) or neoadjuvant chemoradiation alone (control group). Radical surgeries were scheduled after neoadjuvant treatments. The primary endpoint was the pCR rate. The pCR rates were 27.1%, 32.7% and 14.0% for experiment groups A and B and the control group, respectively. The difference in pCR rates between experiment group B and the control group reached statistical significance (risk ratio 2.332, 95% confidence interval 1.106–4.916; P = 0.019). No substantial differences between either one of the experiment groups and the control group were observed regarding adverse reaction, surgical complication and disease progression. Our results show that adding PD-1 blockade after neoadjuvant chemoradiation increases the pCR rate for patients with LARC and raises no substantial safety concerns. Phase 3 trials with larger sample sizes are warranted (ClinicalTrials.gov identifier NCT05245474 ). In a multicenter, open-label, randomized phase 2 trial, neoadjuvant chemoradiation with PD-1 blockade elicited a pathological complete response rate superior to that with neoadjuvant chemoradiation alone in patients with locally advanced rectal cancer.

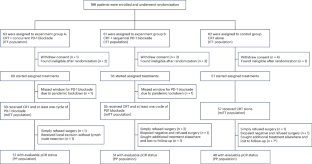
局部晚期直肠癌伴或不伴PD-1阻断的新辅助放化疗:一项随机2期试验
放疗与免疫检查点抑制剂显示出独特的抗肿瘤协同作用,这是由局部晚期直肠癌(LARC)单臂试验的高病理完全缓解(pCR)率所表明的。在此,我们在北京8个主要结直肠癌中心进行了一项2期随机试验,测试了辐射-免疫检查点抑制剂联合治疗LARC患者的有效性和安全性。总共有186名符合条件的参与者(熟练错配修复和缺陷错配修复)被招募。患者被随机分配接受新辅助放化疗+同步/顺序PD-1阻断治疗(实验组A/B组)或单独新辅助放化疗(对照组)。新辅助治疗后安排根治性手术。主要终点为pCR率。实验A、B组和对照组的pCR率分别为27.1%、32.7%和14.0%。实验B组与对照组的pCR率差异有统计学意义(风险比2.332,95%可信区间1.106 ~ 4.916;p = 0.019)。实验组与对照组在不良反应、手术并发症和疾病进展方面均无显著差异。我们的研究结果表明,在新辅助放化疗后添加PD-1阻断剂可以增加LARC患者的pCR率,并且不会引起实质性的安全性问题。iii期临床试验需要更大样本量(ClinicalTrials.gov识别码NCT05245474)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


