Multimodal transcriptomics reveal neurogenic aging trajectories and age-related regional inflammation in the dentate gyrus
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
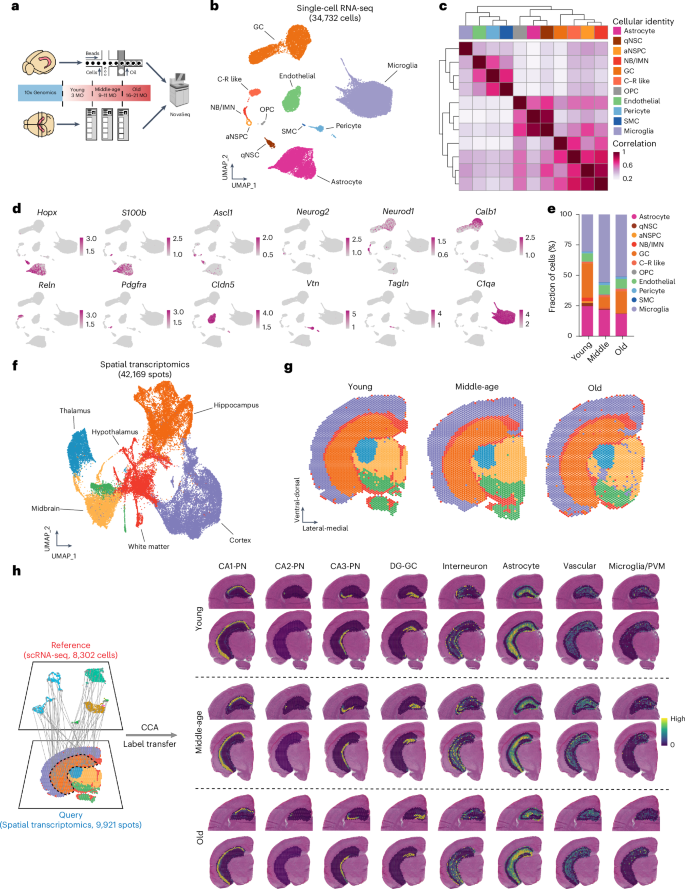
The mammalian dentate gyrus (DG) is involved in certain forms of learning and memory, and DG dysfunction has been implicated in age-related diseases. Although neurogenic potential is maintained throughout life in the DG as neural stem cells (NSCs) continue to generate new neurons, neurogenesis decreases with advancing age, with implications for age-related cognitive decline and disease. In this study, we used single-cell RNA sequencing to characterize transcriptomic signatures of neurogenic cells and their surrounding DG niche, identifying molecular changes associated with neurogenic aging from the activation of quiescent NSCs to the maturation of fate-committed progeny. By integrating spatial transcriptomics data, we identified the regional invasion of inflammatory cells into the hippocampus with age and show here that early-onset neuroinflammation decreases neurogenic activity. Our data reveal the lifelong molecular dynamics of NSCs and their surrounding neurogenic DG niche with age and provide a powerful resource to understand age-related molecular alterations in the aging hippocampus. Multimodal transcriptomics unveil the molecular dynamics of neural stem cells and their surrounding niche in the aging mouse hippocampus and provide a resource to understand age-related molecular changes.

多模态转录组学揭示了齿状回的神经源性衰老轨迹和与年龄相关的区域炎症
哺乳动物齿状回(DG)参与某些形式的学习和记忆,DG功能障碍与年龄相关疾病有关。尽管随着神经干细胞(NSCs)不断产生新的神经元,DG的神经发生潜力在整个生命过程中都保持着,但神经发生随着年龄的增长而减少,这意味着与年龄相关的认知能力下降和疾病。在这项研究中,我们使用单细胞RNA测序来表征神经源性细胞及其周围DG生态位的转录组特征,鉴定与神经源性衰老相关的分子变化,从静止的NSCs的激活到命运决定的后代的成熟。通过整合空间转录组学数据,我们确定了炎症细胞随着年龄的增长进入海马体的区域入侵,并在这里表明早发性神经炎症会降低神经源性活动。我们的数据揭示了NSCs及其周围神经源性DG生态位随年龄的终身分子动力学,并为了解衰老海马中与年龄相关的分子改变提供了强有力的资源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


