The epitranscriptional factor PCIF1 orchestrates CD8+ T cell ferroptosis and activation to control antitumor immunity
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
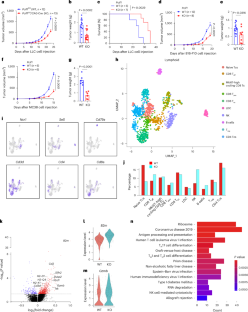
T cell-based immunotherapies have revolutionized cancer treatment, yet durable responses remain elusive. Here we show that PCIF1, an RNA N6 2′-O-dimethyladenosine (m6Am) methyltransferase, negatively regulates CD8+ T cell antitumor responses. Whole-body or T cell-specific Pcif1 knockout (KO) reduced tumor growth in mice. Single-cell RNA sequencing shows an increase in the number of tumor-infiltrating cytotoxic CD8+ T cells in Pcif1-deficient mice. Mechanistically, proteomic and m6Am-sequencing analyses pinpoint that Pcif1 KO elevates m6Am-modified targets, specifically ferroptosis suppressor genes (Fth1, Slc3a2), and the T cell activation gene Cd69, imparting resistance to ferroptosis and enhancing CD8+ T cell activation. Of note, Pcif1-deficient mice had enhanced responses to anti-PD-1 immunotherapy, and Pcif1 KO chimeric antigen receptor T cells improved tumor control. Clinically, cancer patients with low PCIF1 expression in T cells have enhanced responses to immunotherapies. These findings suggest that PCIF1 suppresses CD8+ T cell activation and targeting PCIF1 is a promising strategy to boost antitumor immunity. Here the authors show that PCIF1 can regulate CD8+ T cell antitumor responses in mice and use this information to enhance chimeric antigen receptor T cell design.

表转录因子PCIF1协调CD8+ T细胞的铁凋亡和激活,以控制抗肿瘤免疫
基于T细胞的免疫疗法已经彻底改变了癌症治疗,但持久的反应仍然难以捉摸。在这里,我们发现PCIF1,一种RNA N6 2 ' - o -二甲基腺苷(m6Am)甲基转移酶,负调控CD8+ T细胞抗肿瘤反应。全身或T细胞特异性Pcif1敲除(KO)可降低小鼠肿瘤生长。单细胞RNA测序显示pcif1缺陷小鼠肿瘤浸润性细胞毒性CD8+ T细胞数量增加。在机制上,蛋白质组学和m6am测序分析指出,Pcif1 KO提高了m6am修饰的靶标,特别是铁死亡抑制基因(Fth1, Slc3a2)和T细胞激活基因Cd69,从而增强了对铁死亡的抗性并增强了CD8+ T细胞的激活。值得注意的是,Pcif1缺陷小鼠对抗pd -1免疫治疗的反应增强,Pcif1 KO嵌合抗原受体T细胞改善了肿瘤控制。临床上,T细胞PCIF1低表达的癌症患者对免疫疗法的反应增强。这些发现表明PCIF1抑制CD8+ T细胞的激活,靶向PCIF1是一种很有希望的增强抗肿瘤免疫的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
文献相关原料
公司名称
产品信息
索莱宝
Ficoll-Hypaque density gradient centrifugation
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


