Achieving efficient photoreduction of CO2 by simultaneously facilitating the splitting of H2O and the conversion of *CO intermediates
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
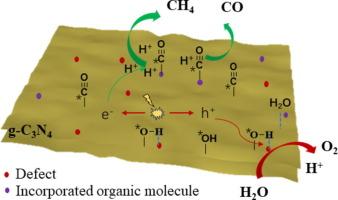
Photocatalytic reduction of CO2 with H2O to hydrocarbon fuels is considered a promising approach to address environmental and energy challenges. However, the efficiency of CO2 photoreduction remains unsatisfactory due to limitations in the reaction rates of CO2 and H2O. This study focuses on the development of defect and organic molecule (3,5-Diamino-1,2,4-triazole, DATZ) modified carbon nitride catalysts (g-CN-DZT) for enhancing the photoreduction of CO2 with H2O. The introduction of defect structures could disrupt the hydrogen bonds and alters the adsorption behavior of OH groups, leading to improved H2O splitting kinetics. Additionally, the incorporation of the organic molecule DATZ facilitates the conversion of *CO intermediates to CO, enhancing the overall CO2 conversion rate. The modified catalyst g-CN-DZ625 exhibits enhanced CO2 capture ability. Meanwhile, N defect serves as an active site for CH4 production. Experimental results show that g-CN-DZ625 demonstrates CO and CH4 evolution rate of 11.86 and 4.45 μmolg-1h−1, respectively, that is 3.62 and 18.54 times higher than that of g-CN-625. The reaction mechanism was further elucidated through in-situ FTIR and DFT simulations.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


