Detailing the activity and deactivation of supported Pt-Sn and Pt-In catalysts for CO2-assisted PDH
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Pt:Sn/MgAl2O4 and Pt:In/MgAl2O4 catalysts with 3 wt% Pt and 3 wt% Sn or In were tested for CO2-assisted propane dehydrogenation (CO2-PDH). This reaction was referenced against Reverse water–gas shift (RWGS) and non-oxidative PDH, while CO-DRIFTS detailed the nanoparticle (NP) surface. Results were connected to a preceding study, focused on NP material properties using Quick X-ray Absorption Spectroscopy and Temporal Analysis of Products. During CO2-PDH, CO2 was mainly consumed through RWGS. CO2 consumption beyond the RWGS equilibrium arose from carbon oxidation. Promoter oxidation by CO2 and the consequent loss of the Pt-based alloys induced carbon formation. This deactivation increased the in situ CO2:H2 ratio, further accelerating promoter oxidation and carbon formation. Repeated catalyst regeneration cycles increased the promoter concentration in the alloyed NPs. This occurred throughout the entire Pt:Sn NP, while for Pt:In, it was limited to the NP surface with concomitant NP bulk depletion. This promoter enrichment contributed to catalyst deactivation.
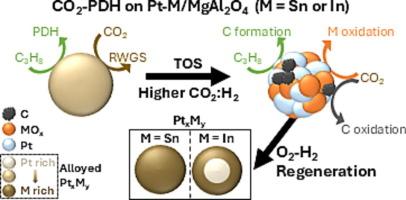
详细介绍了负载Pt-Sn和Pt-In催化剂对co2辅助PDH的活性和失活
以Pt:Sn/MgAl2O4和Pt:In/MgAl2O4为催化剂,分别以3 wt% Pt和3 wt% Sn或In为催化剂,对co2辅助丙烷脱氢(CO2-PDH)进行了试验。该反应参考了逆水气转换(RWGS)和非氧化PDH,而CO-DRIFTS详细描述了纳米颗粒(NP)表面。结果与之前的研究相关联,重点是使用快速x射线吸收光谱和产品时间分析来研究NP材料的特性。在CO2- pdh过程中,CO2主要通过RWGS消耗。超过RWGS平衡的CO2消耗是由碳氧化引起的。促进剂被CO2氧化和随之而来的pt基合金的损失诱导了碳的形成。这种失活增加了原位CO2:H2比,进一步加速了促进剂的氧化和碳的形成。重复的催化剂再生循环增加了合金NPs中的促进剂浓度。这种情况发生在整个Pt:Sn NP中,而对于Pt:In,它仅限于NP表面,并伴随NP体损耗。启动子富集导致催化剂失活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


