Biomimetic Design of Biocompatible Neural Probes for Deep Brain Signal Monitoring and Stimulation: Super Static Interface for Immune Response-Enhanced Contact
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
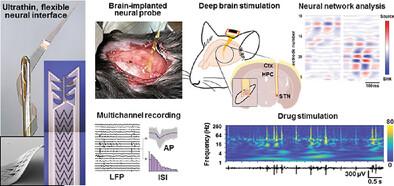
The ability to measure changes in neural activities using devices implanted in the brain can be useful for recording brain signals to assess specific risk factors, monitor the development of brain diseases, and expand the understanding of neural circuitry. Here, a neuroimplantable interface is introduced that integrates biomaterials with an advanced structural design to facilitate monitoring of electrophysiological responses in widespread brain regions. The neural interface uses biocompatible and photopatternable materials to create ultrathin, homogeneous encapsulant/substrate laminates. Comprehensive in vitro tests of the laminin-enveloped neural interface demonstrate efficacy in relieving inflammation via a biomimetic strategy by diminishing microglia and astrocyte aggregation near recording sites, enhancing periodic signal acquisition. The performance is evaluated by injecting an acetylcholine receptor agonist into mouse brains. This approach enables to monitor real-time signal changes, gain insights into neural network dynamics by assessing stimulus-evoked signaling at specific sites, and identify signaling patterns and hippocampal synaptic connections. Additionally, in a Parkinson's disease mouse model, deep brain stimulation is performed and signals are recorded to confirm symptom amelioration, offering a biomedical device approach. The key strategy highlights intact neural electrodes with biocompatible, mechanically compliant materials conferring compact bioelectronic functionalities, high neuronal microenvironment compatibility, and pathological neural system recognition.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


