Age-associated interplay between zinc deficiency and Golgi stress hinders microtubule-dependent cellular signaling and epigenetic control
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Golgi abnormalities have been linked to aging and age-related diseases, yet the underlying causes and functional consequences remain poorly understood. This study identifies the interaction between age-associated zinc deficiency and Golgi stress as a critical factor in cellular aging. Senescent Golgi bodies from human fibroblasts show a fragmented Golgi structure, associated with a decreased interaction of the zinc-dependent Golgi-stacking protein complex Golgin45-GRASP55. Golgi stress is increased, and functions such as glycosylation and vesicle transport are impaired. These disturbances promote Golgi and perinuclear microtubule disassembly and subsequent mislocalization of intracellular proteins associated with cellular signaling and epigenetic control. Pharmacological induction of Golgi stress or zinc deficiency, or ablation of the Golgi-associated zinc transporter gene Zip13 in mouse fibroblasts, replicate the characteristics of cellular senescence, emphasizing the critical role of Golgi-zinc homeostasis. These findings highlight the importance of adequate zinc intake and suggest targeting Golgi dysfunction as a therapeutic strategy for alleviating age-related cellular decline.
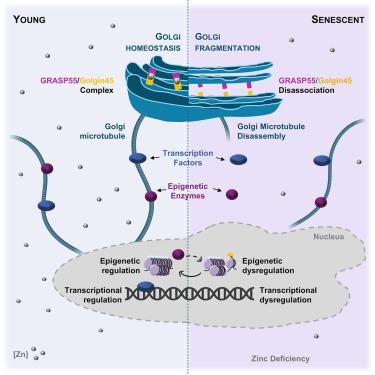
锌缺乏和高尔基应激之间年龄相关的相互作用阻碍了微管依赖的细胞信号传导和表观遗传控制
高尔基体异常与衰老和与年龄相关的疾病有关,但其潜在原因和功能后果仍然知之甚少。这项研究确定了与年龄相关的锌缺乏和高尔基应激之间的相互作用是细胞衰老的关键因素。来自人类成纤维细胞的衰老高尔基体显示出破碎的高尔基结构,这与锌依赖性高尔基堆积蛋白复合物Golgin45-GRASP55的相互作用减少有关。高尔基应激增加,糖基化和囊泡运输等功能受损。这些干扰促进高尔基体和核周微管的解体以及随后与细胞信号传导和表观遗传控制相关的细胞内蛋白的错误定位。高尔基应激或锌缺乏的药物诱导,或小鼠成纤维细胞中高尔基相关锌转运基因Zip13的消融,复制了细胞衰老的特征,强调了高尔基锌稳态的关键作用。这些发现强调了充足的锌摄入的重要性,并建议针对高尔基体功能障碍作为缓解年龄相关细胞衰退的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


