Silanediol-Bay-Bridge Rigidified Axially Chiral Perylene Bisimide
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Chiral organic molecules with a complementing π-structure are highly desired to obtain materials with good semiconducting properties and pronounced chirality effects in the visible region. Herein, we introduce a novel design strategy to achieve an axially chiral and rigid perylene bisimide (PBI) dye by attaching the chirality-inducing 2,2′-biphenoxy moiety at one side of the bay area and the rigidity-inducing di-tert-butylsilanediol bridge on the other side. This yielded a new bay-functionalized PBI derivative carrying the combination of a highly rigid and, simultaneously, an axially chiral perylene core. As a result, the derivative exhibits well-resolved absorption and emission spectra in the visible region, with a fluorescence quantum yield close to unity. Furthermore, the M- and P-enantiomers were found to be stable with a racemization barrier of 102 kJ mol–1 and, hence, could be successfully separated by chiral chromatography and studied by circular dichroism (CD) spectroscopy. This rigidified chiral-PBI could also be crystallized and analyzed by X-ray diffraction, showing the highest torsion angle of the perylene core with a value of up to 30.3° in the family of PBIs carrying the same di-tert-butylsilanediol bridge.
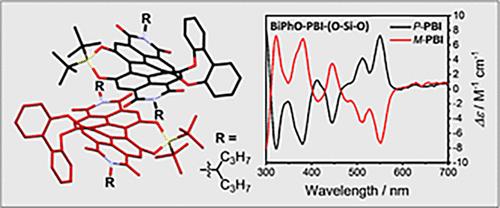
硅二醇-湾桥刚性轴向手性苝双亚胺
人们迫切需要具有互补π结构的手性有机分子来获得具有良好半导体性能和明显可见区手性效应的材料。本文介绍了一种新颖的设计策略,通过在海湾区域的一侧连接诱导手性的2,2 ' -联苯氧基部分,在另一侧连接诱导刚性的二叔丁基硅二醇桥,来实现轴向手性和刚性的苝酰二亚胺(PBI)染料。这产生了一种新的海湾功能化的PBI衍生物,它同时具有高刚性和轴向手性苝核心。结果表明,该衍生物在可见光区表现出良好的吸收和发射光谱,荧光量子产率接近于1。此外,M-和p -对映体在102 kJ mol-1的消旋势垒下是稳定的,因此可以通过手性色谱和圆二色性(CD)光谱成功分离。该刚性手性pbi还可以通过x射线衍射进行结晶和分析,表明在携带相同二叔丁基硅二醇桥的pbi家族中,苝核心的扭转角最高,可达30.3°。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


