Targeted Covalent Nanodrugs Reinvigorate Antitumor Immunity and Kill Tumors via Improving Intratumoral Accumulation and Retention of Doxorubicin
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
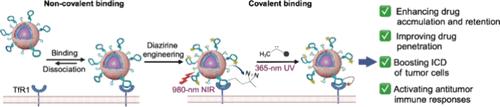
Specifically improving the intratumoral accumulation and retention and achieving the maximum therapeutic efficacy of small-molecule chemotherapeutics remains a considerable challenge. To address the issue, we here reported near-infrared (NIR) irradiation-activatable targeted covalent nanodrugs by installing diazirine-labeled transferrin receptor 1 (TfR1)-targeted aptamers on PEGylated phospholipid-coated upconversion nanoparticles followed by doxorubicin loading. Targeted covalent nanodrugs recognized and then were activated to covalently cross-link with TfR1 on cancer cells by 980 nm NIR irradiation. Systematic studies revealed that they achieved >6- and >5.5-fold higher intratumoral accumulations of doxorubicin than aptamer-based targeted nanodrugs at 6 and 120 h post intravenous injection, respectively. Based on high drug delivery efficacy, targeted covalent nanodrugs boosted doxorubicin-induced immunogenic cell death, activated antitumor immune responses and shrank the sizes of both primary and distant tumors, and displayed better therapeutic efficacy and less adverse effect than targeted nanodrugs and commercial Doxil in 4T1 syngeneic breast tumor model featuring an immunosuppressive microenvironment. By integrating the specificity of molecular recognition, the reactivity profile of diazirine and the accuracy of light manipulation with nanodrug supremacy, our targeted covalent nanodrugs could be expected as a longer-term and efficient strategy to improve anticancer therapeutic efficacy of chemotherapeutics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


