Patterned Antigens on DNA Origami Controls the Structure and Cellular Uptake of Immune Complexes
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
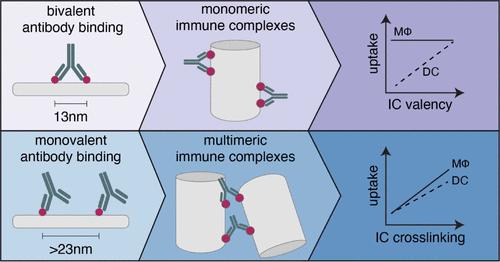
Immune complexes (ICs), formed via antibody (Ab)–antigen (Ag) binding, trigger diverse immune responses, which are critical for natural immunity and have uses for vaccines and immunotherapies. While IC-elicited immune responses depend on its structure, existing methods for IC synthesis produce heterogeneous assemblies, which limits control over their cellular interactions and pharmacokinetics. In this study, we demonstrate the use of DNA origami to create synthetic ICs with defined shape, size, and solubility by displaying Ags in prescribed spatial patterns. We find that Ag arrangement relative to the spatial tolerance of IgG Fab arms (∼13–18 nm) determines IC formation into “monomeric” versus “multimeric” regimes. When Ag spacing matches Fab arm tolerance, ICs are exclusively monomeric, while spacing mismatches favor the formation of multimeric ICs. Within each IC regime, parameters such as the number of Ags and Ab-Ag ratios, as well as DNA origami shape, further fine-tune IC size, shape, and Fc valency. These parameters influenced IC interactions with FcγR-expressing immune cells, with uptake by macrophages showing greater sensitivity to IC cross-linking while dendritic cells were more responsive to Ab valency. Our findings thus provide design principles for controlling the structure and cellular interactions of synthetic ICs and highlight DNA origami-scaffolded ICs as a programmable platform for investigating IC immunology and developing FcγR-targeted therapeutics and vaccines.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


