Functionalized regioisomers of the natural product phenazines myxin and iodinin as potent inhibitors of Mycobacterium tuberculosis and human acute myeloid leukemia cells
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
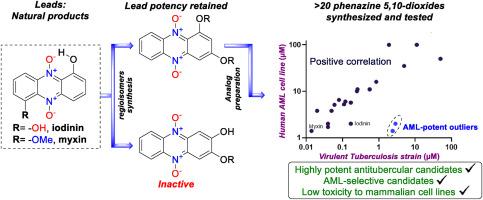
The natural bioactive products myxin and iodinin are phenazine 5,10-dioxides possessing potent anti-bacterial and anti-cancer activity in vitro. This work describes the synthesis and derivatization of new myxin and iodinin regioisomers, developed from 1,3-dihydroxyphenazine 5,10-dioxide. Compounds were evaluated for activity towards M. tuberculosis (Mtb) strains, a human AML cell line (MOLM-13), and two non-cancerous mammalian cell lines (NRK and H9c2). Highly potent analogs were developed having IC50 values against MTB down to 20 nM and 1.4 μM for human AML cells. 1-OH-3-O-alkyl substituted derivatives demonstrated high efficacy against Mtb and low toxicity in normal cells. 2,3-substituted regioisomers of myxin and iodinin were shown to be inactive, highlighting the importance of oxygen substituent in position 1 of the scaffold. A strong positive correlation between anti-MTB and anti-AML activity was revealed, suggesting a common mechanism of action in bacteria and cancer cells. These findings demonstrate the therapeutic potential of 1,3-O-functionalized phenazine 5,10-dioxides in chemotherapy for Mtb and AML and contribute to the structure-activity understanding of phenazine 5,10-dioxides with respect to their biological activity.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


