Cobalt doping regulated Ag electronic structure for boosting electroreduction of CO2 to CO at gas-solid interface
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Electroreduction of gas-phase CO2 (CO2RR) to CO is an attractive way to promote sustainable and carbon-neutral economic development. The Ag-based catalysts have demonstrated great potential for CO2RR to CO reaction. Herein, we showed that the Co-doping into Ag catalyst significantly enhanced the performance of CO2RR. Compared with pure Ag catalyst, the FECO for Ag-Co bimetallic catalyst was increased by nearly 30%, reaching 95.3% with high stability at the full-cell potential of −2.1 V in the gas-phase electroreduction system. Based on physicochemical characterizations, we confirmed that the specific Ag-Co bimetallic structure was formed, the interaction between Ag and Co metals increased the electrochemically active surface area and exposed more adsorption and reactive sites. Combined with the DFT calculation results, we revealed that the Co doping regulated the electronic structure around Ag sites, and more electrons transferred from Co to Ag sites, promoting the CO2 adsorption and the formation of key intermediates (*COOH) on catalyst surface, therefore leading to a higher performance for CO2RR to CO.
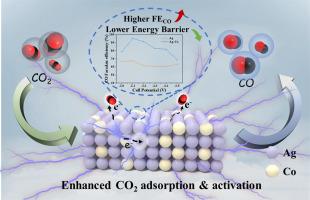
钴掺杂调节Ag电子结构,促进CO2在气固界面电还原为CO
电还原气相CO2 (CO2RR)为CO是促进经济可持续和碳中和发展的一种有吸引力的途径。ag基催化剂在CO2RR - CO反应中表现出很大的潜力。在此,我们发现在Ag催化剂中共掺杂可以显著提高CO2RR的性能。与纯Ag催化剂相比,Ag- co双金属催化剂的FECO提高了近30%,达到95.3%,并且在气相电还原体系中在−2.1 V的满电池电位下具有很高的稳定性。基于理化表征,我们证实了Ag-Co双金属结构的形成,Ag和Co金属之间的相互作用增加了电化学活性表面积,暴露出更多的吸附和反应位点。结合DFT计算结果,我们发现Co掺杂调节了Ag位点周围的电子结构,更多的电子从Co转移到Ag位点,促进了CO2的吸附和催化剂表面关键中间体(*COOH)的形成,从而导致CO2RR对Co的性能更高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


