Pentafluoroorthotellurate Uncovered: Theoretical Perspectives on an Extremely Electronegative Group
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
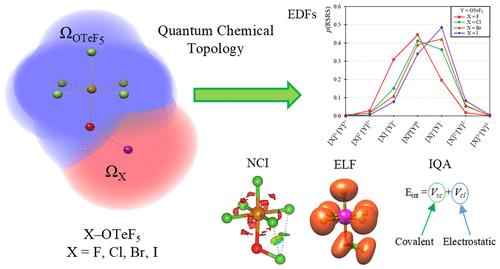
The pentafluoroorthotellurate group (−OTeF5, teflate) exhibits high electron-withdrawing properties. Indeed, it is often used as a bulky substitute for fluoride due to its high chemical stability and larger size, which reduces its tendency to act as a bridging ligand. These characteristics make it a valuable ligand in synthetic chemistry, facilitating the preparation of molecular structures analogous to polymeric fluoride-based compounds. In this study, we explore the electronic structure of the teflate group by using advanced Quantum Chemical Topology (QCT) methods to better understand its bonding nature and compare its group electronegativity with that of the halogens. For that, we examine XOTeF5 systems (X = F, Cl, Br, I) and decompose X–OTeF5 interactions into classical (ionic) and exchange-correlation (covalent) contributions by using interacting quantum atoms (IQA) energy decomposition scheme. We also conduct a detailed analysis of electron distribution by utilizing the statistical framework of electron distribution functions (EDFs) and examine the electron localization function (ELF), electron density, and reduced density gradient scalar functions, as well as delocalization indices and QTAIM charges. The results show that the electron-withdrawing properties of the teflate group are comparable to those of fluorine, albeit slightly lower. Moreover, its internal bonding is primarily ionic. Additionally, we compare −OTeF5 with other O-donor groups, demonstrating that the electron-withdrawing properties within OEF5 (E = S, Se, Te) systems are nearly identical, and these groups show a higher group electronegativity than OCF3, OC(CF3)3, and OC6F5.
未发现的五氟正碲酸盐:一个极电负性基团的理论观点
五氟正碲酸盐基团(- OTeF5, teflate)表现出高的吸电子性能。事实上,它经常被用作氟化物的笨重替代品,因为它具有较高的化学稳定性和更大的尺寸,这降低了它作为桥接配体的倾向。这些特性使其成为合成化学中一种有价值的配体,有助于制备类似于聚合物氟基化合物的分子结构。在这项研究中,我们利用先进的量子化学拓扑(QCT)方法来探索teflate基团的电子结构,以更好地了解其成键性质,并将其与卤素的基团电负性进行比较。为此,我们研究了XOTeF5体系(X = F, Cl, Br, I),并通过相互作用量子原子(IQA)能量分解方案将X - otef5相互作用分解为经典(离子)和交换相关(共价)贡献。我们还利用电子分布函数(edf)的统计框架对电子分布进行了详细的分析,并考察了电子局域函数(ELF)、电子密度、密度梯度标量函数以及离域指数和QTAIM电荷。结果表明,teflate基团的吸电子性能与氟相当,尽管略低。此外,它的内部键主要是离子键。此外,我们将- OTeF5与其他o -供体基团进行了比较,表明OEF5 (E = S, Se, Te)体系中的吸电子性质几乎相同,并且这些基团比OCF3, OC(CF3)3和OC6F5具有更高的基团电负性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


