Xanthine Nucleosides with Pyrazolo[3,4-d]pyrimidine Skeleton: Functionalization with Halogen Atoms, Clickable Side Chains, Pyrene, and iEDDA Cycloadducts, and Impact of Ionic Forms on Photophysical Properties
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
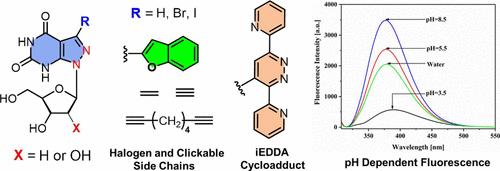
Xanthine nucleosides play a significant role in the expansion of the four-letter genetic code. Herein, 7-functionalized 8-aza-7-deazaxanthine ribo- and 2′-deoxyribonucleosides are described. 2-Amino-6-alkoxy nucleosides were converted to halogenated 8-aza-7-deazaxanthine nucleosides by deamination followed by hydroxy/alkoxy substitution. 8-Aza-7-deaza-7-iodo-2′-deoxyxanthosine served as an intermediate in Suzuki–Miyaura, Sonogashira, and Stille reactions. Alkynyl and vinyl side chains as well as fluorescent tags were introduced. Pyrene conjugates were derived by copper(I)-catalyzed cycloaddition. Inverse-electron-demand Diels–Alder reaction of 8-aza-7-deaza-7-vinyl-2′-deoxyxanthosine with 3,6-dipyridyl-tetrazine proceeded with a second-order rate constant of 0.042 L M–1 s–1. X-ray analysis of 8-aza-7-deaza-7-vinyl-2′-deoxyxanthosine displayed two conformers with a syn conformation. Crystal packing is stabilized by xanthine base pairs. UV spectroscopy confirmed the sensitivity of 7-functionalized 8-aza-7-deazaxanthine nucleosides to pH changes. Halogen and alkynyl substituents decrease pK values, and vinyl, pyrene, or benzofuran leads to an increase. Fluorescence measurements of 8-aza-7-deaza-7-benzofuran-2′-deoxyxanthosine disclosed solvatochromism and enhanced fluorescence when the pH or the viscosity of the solvent is increased. Nucleoside pyrene conjugates connected by a linear linker displayed monomer emission, and two pyrene residues connected by a dendritic linker exhibited excimer emission. According to their fluorescence properties and sensitivity to pH changes, the functionalized 8-aza-7-deazaxanthine nucleosides expand the class of nucleosides applicable to fluorescence detection for diagnostic and therapeutic purposes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


