Bioremediation of catecholic pollutants with novel oxygen-insensitive catechol 2,3-dioxygenase and its potential in biomonitoring of catechol in wastewater
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The oxygenases are essential in the bioremediation of xenobiotic pollutants. To overcome cultivability constraints, this study aims to identify new potential extradiol dioxygenases using the functional metagenomics approach. RW1-4CC, a novel catechol 2,3-dioxygenase, was isolated using functional metagenomics approach, expressed in a heterologous system, and characterized thoroughly using state-of-the-art techniques. The serial truncation mutations of the C-terminal tail increase the catalytic efficiency of truncated proteins against the 2,3-dihydroxybiphenyl (2,3-DHB). RW1-4CC lose its 50% of activity at 60 °C, with its optimum temperature at 15 °C, whereas the truncated proteins were found to be more stable at extended temperature range, i.e., both RW1-4CC-A and RW1-4CC-B retained 50% of their activity at 75 °C, with their temperature optima at 55 °C and 65 °C, respectively. The molecular docking studies further confirmed the high binding affinity of truncated proteins for the 2,3-DHB than catechol. The molecular modeling analysis revealed the difference in iron-binding and substrate interacting environment of RW1-4CC and its truncated proteins. The efficiency of purified RW1-4CC to detect catechol was evaluated using a gold screen-printed electrode by cyclic voltammetry. RW1-4CC detected catechol in wastewater and artificial seawater up to the concentration of 100 μm, which makes it reliable for catechol detection.

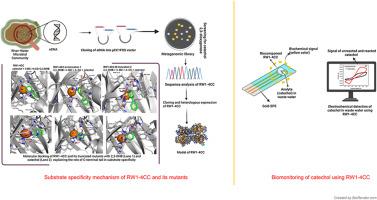
新型对氧不敏感的儿茶酚2,3-双加氧酶对儿茶酚污染物的生物修复及其在废水中儿茶酚生物监测中的应用前景
加氧酶在生物修复外源污染物中起着至关重要的作用。为了克服可培养性的限制,本研究旨在利用功能宏基因组学方法鉴定新的潜在的外二醇双加氧酶。RW1-4CC是一种新型的儿茶酚2,3-双加氧酶,采用功能元基因组学方法分离,在异源系统中表达,并使用最先进的技术进行了彻底的表征。c端尾部的连续截断突变增加了截断蛋白对2,3-二羟基联苯(2,3- dhb)的催化效率。RW1-4CC在60°C时失去50%的活性,其最适温度为15°C,而截断的蛋白在更大的温度范围内更稳定,即RW1-4CC- a和RW1-4CC- b在75°C时保持50%的活性,其最适温度分别为55°C和65°C。分子对接研究进一步证实了截断蛋白对2,3- dhb的结合亲和力高于儿茶酚。分子模型分析显示RW1-4CC及其截断蛋白的铁结合和底物相互作用环境存在差异。用循环伏安法评价了纯化后的RW1-4CC在金丝网印刷电极上检测儿茶酚的效率。RW1-4CC检测废水和人工海水中儿茶酚的浓度可达100 μm。为儿茶酚的检测提供了可靠的依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


