Synergistic Solvation and Nucleation Regulation for Enhanced Stability and Longevity in Aqueous Zinc-Ion Batteries with d-Pantothenic Acid Additive
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aqueous zinc-ion batteries (AZIBs) have gained increasing attention for grid energy storage systems. However, ensuring the long-term reversible operation of the zinc anode remains a challenge due to dendrite growth and adverse side reactions during the charge and discharge cycles. This study investigates the use of d-pantothenic acid (D-PA) as an additive in 2 M ZnSO4 aqueous electrolyte to enhance the cycling stability of the zinc anode in AZIBs. Experimental results and theoretical calculations demonstrate that D-PA reshapes the solvation structure of Zn2+ by partially replacing coordinated water molecules, ensuring the stability of Zn2+ transport. Furthermore, D-PA adsorbs on active sites of the zinc anode, increasing the surface overpotential (|ηs|), reducing the nucleation energy barrier, and decreasing the critical nucleus size (rcrit), thus ensuring uniform zinc deposition. This dual role of modifying the Zn2+ solvation shell and regulating Zn2+ nucleation effectively mitigates dendrite growth and suppresses side reactions, resulting in excellent stability of the zinc anode. Consequently, Zn||Zn symmetrical cells with the D-PA additive maintain stable operation for over 2000 h at 1.0 mA cm–2 and 1.0 mA h cm–2, and nearly 4000 h at 4.0 mA cm–2 and 4.0 mA h cm–2. Additionally, Zn||Cu asymmetric cells exhibit cycling stability over 300 cycles at 0.5 mA cm–2 and 0.5 mA h cm–2, with an average Coulombic efficiency of 99.29%. Moreover, Zn||V2O5 full cells containing the D-PA additive exhibit stable cycling performance over 1000 cycles at a current density of 1 A g–1, maintaining a high capacity retention. Specifically, the initial capacity of the full cell is around 161.17 mA h g–1, with approximately 62.7% capacity retention after 1000 cycles.
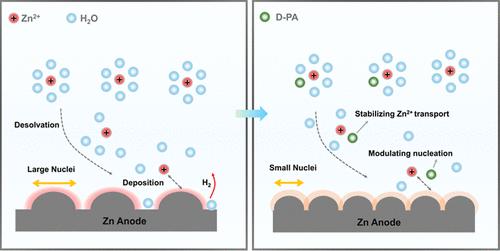
d-泛酸添加剂对提高锌离子电池稳定性和寿命的协同溶剂化和成核调控
水溶液锌离子电池(azib)在电网储能系统中受到越来越多的关注。然而,由于在充放电循环过程中枝晶生长和不良副反应,确保锌阳极的长期可逆运行仍然是一个挑战。本文研究了在2 M氧化锌水溶液中添加d-泛酸(D-PA)以提高AZIBs中锌阳极的循环稳定性。实验结果和理论计算表明,D-PA通过部分取代配位水分子,重塑了Zn2+的溶剂化结构,保证了Zn2+输运的稳定性。此外,D-PA吸附在锌阳极的活性位点,增加了表面过电位(|ηs|),降低了成核能势垒,降低了临界核尺寸(rcrit),从而保证了锌的均匀沉积。这种修饰Zn2+溶剂化壳层和调节Zn2+成核的双重作用,有效地减缓了枝晶的生长,抑制了副反应,使锌阳极具有优异的稳定性。因此,添加了D-PA添加剂的Zn||Zn对称电池在1.0 mA cm-2和1.0 mA h cm-2下可稳定工作2000小时以上,在4.0 mA cm-2和4.0 mA h cm-2下可稳定工作近4000小时。此外,Zn||Cu不对称电池在0.5 mA cm-2和0.5 mA h cm-2下具有300次循环的稳定性,平均库仑效率为99.29%。此外,含有D-PA添加剂的Zn||V2O5全电池在电流密度为1 a g-1的情况下,在1000次循环中表现出稳定的循环性能,保持了较高的容量保持率。具体来说,充满电池的初始容量约为161.17 mA h g-1,在1000次循环后容量保持率约为62.7%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
文献相关原料
公司名称
产品信息
阿拉丁
ZnSO4·7H2O
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


