Targeting JNK3 for Alzheimer's disease: Design and synthesis of novel inhibitors with aryl group diversity utilizing wide pocket
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
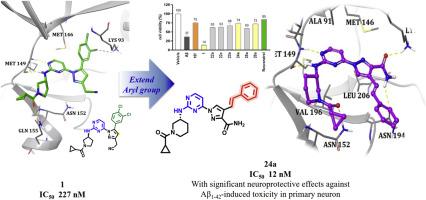
JNK3, a brain-specific stress-activated protein kinase, plays a critical role in Alzheimer's disease pathogenesis through phosphorylation of Tau and APP. This study aimed to develop selective JNK3 inhibitors based on a pyrazole scaffold, focusing on (E)-1-(2-aminopyrimidin-4-yl)-4-styryl-1H-pyrazole-3-carboxamide derivatives. Through systematic structural modifications and extensive SAR analysis, we identified compounds 24a and 26a as highly potent JNK3 inhibitors, with IC50 values of 12 and 19 nM, respectively. Especially, 24a revealed its potent and selective inhibition of JNK3, coupled with inhibition of the GSK3α/β kinases involved in Tau phosphorylation. In vitro studies revealed significant neuroprotective effects against Aβ1-42-induced toxicity in primary neuronal cells and western blot analyses confirmed the compounds' ability to mitigate Aβ1-42-induced c-Jun and APP phosphorylation, suggesting a multi-faceted approach to neuroprotection. Docking studies validated the retention of optimal interactions within the JNK3 binding pocket. Importantly, BBB PAMPA assays and ADME predictions indicated favorable blood-brain barrier permeability and pharmacokinetic profiles for the lead compounds. These findings represent a significant advancement in the development of selective JNK3 inhibitors, providing a strong foundation for further preclinical development of potential Alzheimer's disease therapeutics.

靶向JNK3治疗阿尔茨海默病:设计和合成具有广泛芳基多样性的新型抑制剂
JNK3是一种脑特异性应激激活蛋白激酶,通过Tau和APP的磷酸化在阿尔茨海默病的发病机制中起着至关重要的作用。本研究旨在开发基于吡唑支架的选择性JNK3抑制剂,重点研究(E)-1-(2-氨基嘧啶-4-基)-4-苯基- 1h -吡唑-3-羧酰胺衍生物。通过系统的结构修饰和广泛的SAR分析,我们确定化合物24a和26a是高效的JNK3抑制剂,IC50值分别为12和19 nM。特别是,24a显示其对JNK3的有效和选择性抑制,以及对参与Tau磷酸化的GSK3α/β激酶的抑制。体外研究显示,该化合物对a β1-42诱导的原代神经细胞毒性具有显著的神经保护作用,western blot分析证实了该化合物能够减轻a β1-42诱导的c-Jun和APP磷酸化,表明其具有多方面的神经保护作用。对接研究证实了JNK3结合口袋中保留了最佳相互作用。重要的是,BBB PAMPA分析和ADME预测表明,先导化合物具有良好的血脑屏障通透性和药代动力学特征。这些发现代表了选择性JNK3抑制剂开发的重大进展,为进一步临床前开发潜在的阿尔茨海默病治疗药物提供了坚实的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


