Graphene oxide supported oxygen vacancy-rich Co3O4 and Ni nanoparticle for boosting the hydrogen storage properties of MgH2
IF 15.8
1区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
Developing efficient catalysts is pivotal for advancing MgH2-based hydrogen storage systems. In this study, a novel catalyst, graphene oxide-supported oxygen vacancy-rich Co3O4 and Ni nanoparticles (Ni-OV-C@GO), was synthesized to enhance the hydrogen storage performance of MgH2. The catalyst dramatically improved the kinetics of MgH2, lowering the initial hydrogen desorption temperature of Ni-OV-C@GO-MgH2–7 to 438 K, which is 386 K lower than that of as-milled MgH2. The composite achieved 5.0 wt% hydrogen absorption at 423 K within 600 s and retained 97.3 % capacity after 30 cycles. Notably, the activation energy for H2 desorption was reduced to 40.78 kJ/mol, an 80 % decrease compared to pristine MgH2. The in-situ formation of CoMg2/CoMg2H5 and Mg2Ni/Mg2NiH4 acted as “hydrogen pumps”, facilitating multiple hydrogen transfer pathways. Additionally, oxygen vacancies elongated Mg-H bonds, enhancing dehydrogenation kinetics through catalytic effects. These findings provide valuable insights into improving hydrogen adsorption and desorption kinetics in MgH2-based systems.
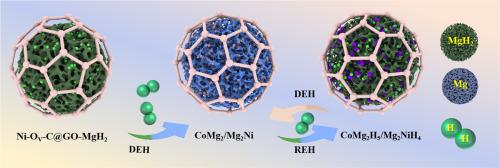
氧化石墨烯负载富氧空位的Co3O4和Ni纳米颗粒,提高MgH2的储氢性能
开发高效催化剂是推进基于mgh2的储氢系统的关键。在本研究中,合成了一种新型催化剂,氧化石墨烯负载的富氧空位Co3O4和Ni纳米颗粒(Ni-OV-C@GO),以增强MgH2的储氢性能。催化剂显著改善了MgH2的动力学,降低了初始氢解吸温度Ni-OV-C@GO-MgH2 -7至438 K,比MgH2的初始氢解吸温度低386 K。在423 K温度下,该复合材料在600秒内的吸氢率达到5.0 wt%, 30次循环后的吸氢率保持在97.3%。值得注意的是,与原始MgH2相比,H2解吸活化能降低到40.78 kJ/mol,降低了80%。CoMg2/CoMg2H5和Mg2Ni/Mg2NiH4的原位形成起到了“氢泵”的作用,促进了多种氢转移途径。此外,氧空位拉长了Mg-H键,通过催化作用增强了脱氢动力学。这些发现为改善mgh2基体系的氢吸附和解吸动力学提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Magnesium and Alloys
Engineering-Mechanics of Materials
CiteScore
20.20
自引率
14.80%
发文量
52
审稿时长
59 days
期刊介绍:
The Journal of Magnesium and Alloys serves as a global platform for both theoretical and experimental studies in magnesium science and engineering. It welcomes submissions investigating various scientific and engineering factors impacting the metallurgy, processing, microstructure, properties, and applications of magnesium and alloys. The journal covers all aspects of magnesium and alloy research, including raw materials, alloy casting, extrusion and deformation, corrosion and surface treatment, joining and machining, simulation and modeling, microstructure evolution and mechanical properties, new alloy development, magnesium-based composites, bio-materials and energy materials, applications, and recycling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


