Fine-tuned coordination environment of Pt-Fe-Pt active site for selective heterogeneous hydrogenation of crotonaldehyde
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Dispersing the catalytically more active noble metal at the single-site scale ensures maximum atom efficiency for selective heterogeneous hydrogenation over bimetallic particles. However, the low density and random location of the noble-metal atoms compromise the intrinsic activity and/or selectivity because of the resulting altered electronic structure. Here, we report that densely populating and precisely arranging Pt atoms in the form of a Pt-Fe-Pt heterotrimer not only catalyzes preferential hydrogenation of the C=O bond in crotonaldehyde (CAL) but also increases the reaction rate by 35-fold, circumventing the activity-selectivity trade-off. The Pt-Fe-Pt active site is fabricated by H2 reduction at 673 K of a Pt-Fe2O3 particle pair, wherein a 3.3 nm Pt particle sits on a 9.8 nm Fe2O3 particle. It interacts with the CAL molecule in a site-bond recognition manner: the left-end Pt atom anchors the C=C bond, whereas the central Fe atom activates the C=O bond, which is further hydrogenated by H atoms adsorbed on the right-end Pt atom.
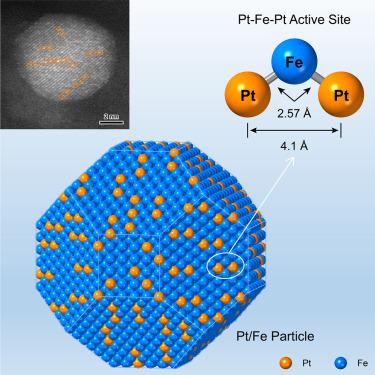
巴豆醛选择性非均相加氢Pt-Fe-Pt活性位点配位环境的微调
将催化活性更高的贵金属分散在单位点尺度上,可确保在双金属颗粒上进行选择性非均相氢化的最大原子效率。然而,由于贵金属原子的低密度和随机位置,导致电子结构改变,从而损害了其固有活性和/或选择性。在这里,我们报道了密集填充和精确排列Pt原子以Pt- fe -Pt异质三聚体的形式不仅催化巴丁醛(CAL)中C=O键的优先氢化,而且还将反应速率提高了35倍,绕过了活性-选择性权衡。在673 K下H2还原Pt-Fe2O3粒子对制备了Pt- fe -Pt活性位点,其中3.3 nm的Pt粒子位于9.8 nm的Fe2O3粒子上。它以位点键识别的方式与CAL分子相互作用:左端Pt原子锚定C=C键,而中心Fe原子激活C=O键,C=O键被吸附在右端Pt原子上的H原子进一步氢化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


