Dehydrogenase versus Oxidase Function: The Interplay between Substrate Binding and Flavin Microenvironment
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
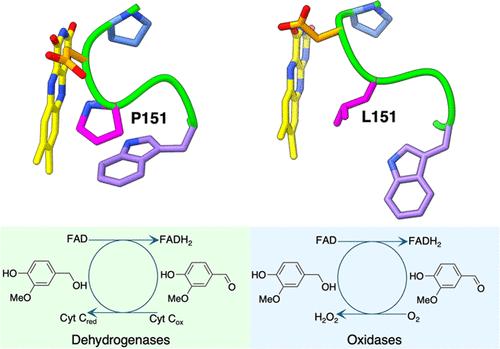
Redox enzymes, mostly equipped with metal or organic cofactors, can vary their reactivity with oxygen by orders of magnitude. Understanding how oxygen reactivity is controlled by the protein milieu remains an open issue, with broad implications for mechanistic enzymology and enzyme design. Here, we address this problem by focusing on a widespread group of flavoenzymes that oxidize phenolic compounds derived from microbial lignin degradation, using either oxygen or cytochrome c as an electron acceptor. A comprehensive phylogenetic analysis revealed conserved amino acid motifs in the flavin-binding site. Using a combination of kinetic, mutagenesis, structural, and computational methods, we examined the role of these residues. Our results demonstrate that subtle and localized changes in the flavin environment can drastically impact oxygen reactivity. These effects are afforded through the creation or blockade of pathways for oxygen diffusion. Substrate binding plays a crucial role by potentially obstructing oxygen access to the flavin, thus influencing the enzyme’s reactivity. The switch between oxidase and dehydrogenase functionalities is thereby achieved through targeted, site-specific amino acid replacements that finely tune the microenvironment around the flavin. Our findings explain how very similar enzymes can exhibit distinct functional properties, operating as oxidases or dehydrogenases. They further provide valuable insights for the rational design and engineering of enzymes with tailored functions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


