New insights into activation mechanisms of peroxymonosulfate by halide ions for micropollutant abatement: The generation routes and contributions of reactive radicals and hypohalous acid
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
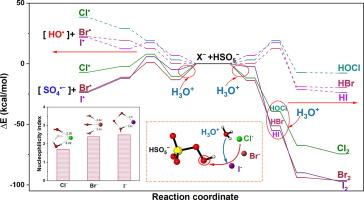
Micropollutants in aquatic environments is a pressing concern due to the risk to ecological and human health, necessitating innovative treatments. Peroxymonosulfate (PMS) activation by halide ions (X− = Cl−, Br−, and I−) was investigated for degrading micropollutants. The PMS/Cl− process could be enhanced by increased H+ and Cl−, not affected by general inorganic ions (NO3−, SO42−, H2PO4−), but inhibited by natural organic matter. In real water matrices, original 5.7 mM Cl− could activate 1 mM PMS to effectively degrade 99 % bisphenol A, achieving substantial reductions in the cytotoxicity. Both single-electron and two-electron transfer routes existed in the PMS/X− reactions, generating radicals and HOX/X2, and Cl−-contained radicals predominantly decomposed micropollutants with electron-withdrawing or methoxy groups. I− with greater nucleophilicity exhibits the highest efficiency at lowest concentration in PMS/X−. This research provides new insights into the PMS/X− system, offering a promising approach for enhancing water treatment to degrade micropollutants in X−-contained scenarios
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


