Polyacetals of higher cyclic formals: synthesis, properties and application as polymer electrolytes†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
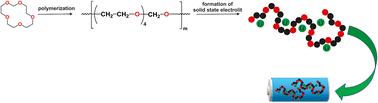
Polymers of higher cyclic formals (polyacetals) containing alternating oxymethylene (OM) units and a few oxyethylene (EO) units can be considered as intermediates between poly(ethylene oxide) and poly(1,3-dioxolane), both of which are used as components in solid polymer electrolytes. In this work, polyacetals from di-, tri-, and tetraethylene glycol cyclic formals (POMEO2, POMEO3, and POMEO4) were obtained with high efficiency (>95%) by cationic polymerization conducted at 20 °C in CH2Cl2, using triethyloxonium hexafluorophosphate as a catalyst. Analogously, polyacetals (POMEOx) of higher cyclic formals of commercially available poly(ethylene oxide) diols (Mn ∼ 200 g mol−1) were prepared under these conditions. The obtained polymers were carefully characterised using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to determine their glass transition temperature (Tg), melting temperature (Tm) and thermal stability. Subsequently, solid-state electrolytes were prepared by applying lithium trifluoromethane sulfonate (lithium triflate –LiOTf). The complexation of the lithium cation was studied by classical 7Li NMR and DOSY techniques. Ionic conductivity measurements of the polyacetal electrolytes were performed using electrochemical impedance spectroscopy (EIS) in the temperature range from 20 to 100 °C. The results show that disrupting the regular structure of polymers obtained by polymerizing cyclic formals of individual diols, by introducing ethylene oxide units of different lengths within a macromolecule, may benefit ionic transport.

高环甲醛聚缩醛:合成、性能及其作为聚合物电解质的应用
含有氧亚甲基(OM)单元和少量氧亚乙基(EO)单元交替出现的高环醛聚合物(聚缩醛)可被视为聚环氧乙烷和聚(1,3-二氧戊环)之间的中间体,这两种聚合物都可用作固体聚合物电解质的成分。在这项研究中,以六氟磷酸三乙氧基铵为催化剂,在 20 °C 的 CH2Cl2 溶液中进行阳离子聚合反应,以高效率(95%)获得了由二、三和四乙二醇环醛(POMEO2、POMEO3 和 POMEO4)组成的聚缩醛。同样,在这些条件下还制备了市售聚环氧乙烷二元醇(Mn ∼ 200 g mol-1)的高环形式的聚醋酸酯(POMEOx)。使用差示扫描量热法(DSC)和热重分析法(TGA)对获得的聚合物进行了仔细的表征,以确定其玻璃化转变温度(Tg)、熔化温度(Tm)和热稳定性。随后,利用三氟甲烷磺酸锂(三氟甲基磺酸锂-LiOTf)制备了固态电解质。通过经典的 7Li NMR 和 DOSY 技术研究了锂阳离子的络合。使用电化学阻抗谱(EIS)测量了聚缩醛电解质在 20 至 100 °C 温度范围内的离子电导率。结果表明,通过在大分子中引入不同长度的环氧乙烷单元,破坏通过聚合单个二元醇的环状形式获得的聚合物的规则结构,可能有利于离子传输。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


