Design, synthesis and biological evaluation of novel 1H-indole-3-carbonitrile derivatives as potent TRK Inhibitors
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Tropomyosin receptor kinase (TRK) has emerged as a promising therapeutic target in cancers driven by NTRK gene fusions. Herein, we report a highly potent TRK inhibitor, C11, developed using bioisosteric replacement and computer-aided drug design (CADD) strategies. Compound C11 demonstrated significant antiproliferative effects against TRK-dependent cell lines (Km-12), and exhibited a dose-dependent inhibition of both colony formation and cell migration. Mechanistic study revealed that C11 induced cancer cell death by arresting the cell cycle, triggering apoptosis, and reducing phosphorylated TRK levels. In vitro stability assays showed that compound C11 possessed excellent plasma stability (t1/2 > 480 min) and moderate liver microsomal stability (t1/2 = 38.9 min). Pharmacokinetic evaluation further indicated an oral bioavailability of 15.2 % for compound C11. These results highlight compound C11 as a promising lead compound for the further development of TRK inhibitors.
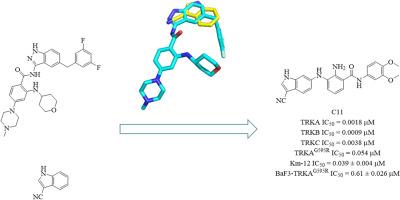

新型强效TRK抑制剂1h -吲哚-3-碳腈衍生物的设计、合成及生物学评价
原肌球蛋白受体激酶(TRK)已成为NTRK基因融合驱动的癌症的一个有希望的治疗靶点。在此,我们报道了一种高效的TRK抑制剂C11,它是用生物等构替代和计算机辅助药物设计(CADD)策略开发的。化合物C11对trk依赖性细胞系(Km-12)具有显著的抗增殖作用,对集落形成和细胞迁移均有剂量依赖性的抑制作用。机制研究表明,C11通过阻滞细胞周期、触发细胞凋亡、降低磷酸化TRK水平诱导癌细胞死亡。体外稳定性实验表明,化合物C11具有良好的血浆稳定性(t1/2 >;肝微粒体稳定性中等(t1/2 = 38.9 min)。药代动力学评价进一步表明,化合物C11的口服生物利用度为15.2%。这些结果突出了化合物C11作为进一步开发TRK抑制剂的有希望的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


