The efflux pump ABCC1/MRP1 constitutively restricts PROTAC sensitivity in cancer cells
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
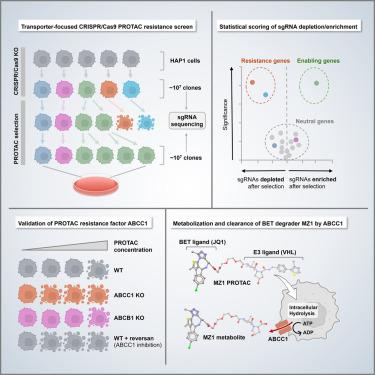
Proteolysis targeting chimeras (PROTACs) are bifunctional molecules that induce selective protein degradation by linking an E3 ubiquitin ligase enzyme to a target protein. This approach allows scope for targeting “undruggable” proteins, and several PROTACs have reached the stage of clinical candidates. However, the roles of cellular transmembrane transporters in PROTAC uptake and efflux remain underexplored. Here, we utilized transporter-focused genetic screens to identify the ATP-binding cassette transporter ABCC1/MRP1 as a key PROTAC resistance factor. Unlike the previously identified inducible PROTAC exporter ABCB1/MDR1, ABCC1 is highly expressed among cancers of various origins and constitutively restricts PROTAC bioavailability. Moreover, in a genome-wide PROTAC resistance screen, we identified candidates involved in processes such as ubiquitination, mTOR signaling, and apoptosis as genetic factors involved in PROTAC resistance. In summary, our findings reveal ABCC1 as a crucial constitutively active efflux pump limiting PROTAC efficacy in various cancer cells, offering insights for overcoming drug resistance.

外排泵ABCC1/MRP1组成性地限制癌细胞中PROTAC的敏感性
蛋白水解靶向嵌合体(Proteolysis targeting chimeras, PROTACs)是一种双功能分子,通过将E3泛素连接酶连接到靶蛋白上,诱导选择性蛋白质降解。这种方法允许靶向“不可药物”的蛋白质,并且一些PROTACs已经达到临床候选阶段。然而,细胞跨膜转运蛋白在PROTAC摄取和外排中的作用仍未得到充分研究。在这里,我们利用以转运蛋白为中心的遗传筛选来鉴定atp结合盒转运蛋白ABCC1/MRP1是一个关键的PROTAC抗性因子。与先前确定的可诱导PROTAC输出者ABCB1/MDR1不同,ABCC1在各种来源的癌症中高度表达,并构成限制PROTAC的生物利用度。此外,在全基因组的PROTAC耐药筛选中,我们发现了参与泛素化、mTOR信号传导和凋亡等过程的候选基因,这些基因都是PROTAC耐药的遗传因素。总之,我们的研究结果表明ABCC1是一个关键的组成型活性外排泵,限制了PROTAC在各种癌细胞中的疗效,为克服耐药提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


